42320-B1
Approaches to the Synthesis of C-Linked Glycosyl Amino Acids
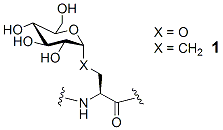
The biochemical understanding of protein glycosylation is making great strides, and interest is expanding in developing a variety of glycoconjugates and their mimics as enzyme inhibitors and as pharmaceutical targets. A simple, economic method for constructing metabolically stable isosteres of O-glycosyl amino acids or peptides would be highly beneficial. Our focus entails altering the nature of the connecting chain between the glycoside and the peptide through a methylene-for-oxygen substitution, namely the formation of C-glycosyl serine, such as 1. C-linked glycosides are robust to degradation by glycosidases, reaction with glycosyltransferases, acid hydrolysis of the former anomeric acetal, and beta-elimination from the serine, and thus offer a platform from which to explore their biochemical impact and pharmaceutical potential.
Although initial advances were described
with a cross-metathesis strategy for the preparation of compounds like 1, we have found advantage in using a
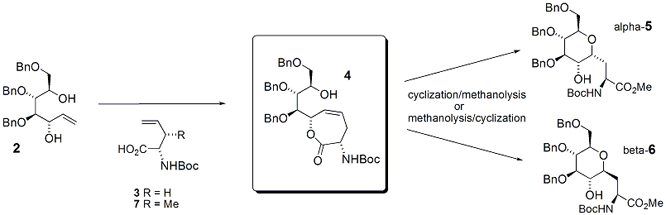
ring-closing metathesis (RCM) strategy. Toward that end, a common intermediate 4 has been prepared from compounds 2 and 3 by esterification and RCM. The esterification step is
unfortunately not selective for the allylic, secondary hydroxyl over the other
secondary hydroxyl, therefore we have resorted to a silyl-protected heptene (not shown) prepared via the dithioacetal of an
arabinose precursor.
With the common intermediate 4 in hand, we have demonstrated that electrophilic cyclization onto the alkene, followed by
methanolysis affords the alpha-isomer 5,
whereas a reversal of these two reactions yields the beta-isomer 6.
Extensions of this work are ongoing to
prepare glycosides other than glucose and to form a C-glycosyl threonine from 7.