

44764-B6
Computational Studies of the Atmospheric Chemistry of Alkene Ozonolysis Intermediates
My laboratory has focused on two projects with PRF support. Both have involved the use of quantum chemistry and statistical rate theory to predict quantities that can be tested against experiment.
(1) Hydroxyl Radical Production from Thermalized Carbonyl Oxides. It is well established that a chemically activated syn carbonyl oxide (1) isomerizes to a vinyl hydroperoxide (3, Reaction 1), which decomposes quantitatively to afford ·OH.
However, the atmospheric fate of
thermalized syn carbonyl oxides is not
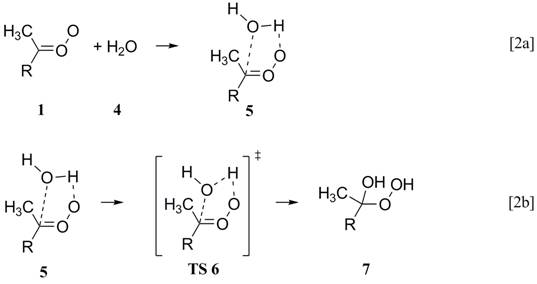
clear, because Reaction 1 will compete with the bimolecular reaction with water
(Reaction 2):
We are using highly accurate
composite electronic structure methods and variational transition state theory
to predict rate constants for Reactions 1 and 2b. Table 1 summarizes MCG3 energetics based on
QCISD/MG3 (or QCISD/6-31G(d)) optimized geometries. Density functional theory always predicts
reaction barriers (not shown) lower than the MCG3//QCISD barrier, although
B3LYP/6-31+G(d,p) typically is within 1
kcal/mol of the higher level barriers.
TABLE 1: Relative Energies (in kcal/mol) for the Species in Reactions 1
and 2 Species R Energy Relative to TS 2 H +17.91 1 TS 2 +16.86 1 TS 2 +18.34 1 3 H -17.62 1 3 -16.48 1 3 -12.12 1 5 H -6.22 1 + 4 5 -6.88 1 + 4 5 -6.09 1 + 4 TS 6 H +15.05 5 TS 6 +14.53 5 TS 6 +15.91 5 7 H -28.42 5 7 -23.38 5 7 -22.21 5 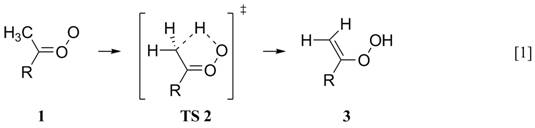


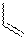

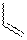

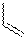

Preliminary
kinetics results, based on B3LYP/6-31+G(d,p) energies, gradients, and Hessians,
are reported for Reaction 1 in Table 2 and for Reaction 2b in Table 3. Rate constants were calculated using
conventional transition state theory (TST) and canonical variational transition
state theory (CVT). All vibrational
modes with frequencies less than 200 cm-1 were treated as hindered
internal rotors. Transmission coefficients were estimated using Truhlar's multidimensional
optimized tunneling method (mOMT) and
by fitting the minimum energy path with the asymmetric Eckart potential
(Eckart).
TABLE 2. Rate Constants and Transmission Coefficients for Reaction 1
| Rate Constant (s-1)
| Transmission Coefficient
| ||
T/K | TST | CVT | mOMT | Eckart |
200 | 2.48E-06 | 2.47E-06 | 1.18E+06 | 3.39E+05 |
250 | 1.19E-02 | 1.19E-02 | 2.18E+03 | 9.25E+02 |
298 | 2.79E+00 | 2.79E+00 | 8.92E+01 | 5.46E+01 |
400 | 3.95E+03 | 3.95E+03 | 6.48E+00 | 6.02E+00 |
600 | 4.81E+06 | 4.81E+06 | 1.99E+00 | 2.21E+00 |
800 | 1.76E+08 | 1.76E+08 | 1.44E+00 | 1.65E+00 |
1000 | 1.58E+09 | 1.58E+09 | 1.25E+00 | 1.44E+00 |
1200 | 6.98E+09 | 6.98E+09 | 1.17E+00 | 1.33E+00 |
1400 | 2.05E+10 | 2.05E+10 | 1.12E+00 | 1.27E+00 |
1600 | 4.66E+10 | 4.66E+10 | 1.09E+00 | 1.22E+00 |
1800 | 8.90E+10 | 8.90E+10 | 1.07E+00 | 1.19E+00 |
2000 | 1.50E+11 | 1.50E+11 | 1.06E+00 | 1.17E+00 |
TABLE 3. Rate Constants and Transmission Coefficients for Reaction 2b
| Rate Constant (s-1)
| Transmission Coefficient
| ||
T/K | TST | CVT | mOMT | Eckart |
200 | 1.99E-03 | 1.31E-03 | 3.07E+00 | 2.17E+00 |
250 | 1.39E+00 | 1.02E+00 | 1.95E+00 | 1.70E+00 |
298 | 9.33E+01 | 7.30E+01 | 1.58E+00 | 1.51E+00 |
400 | 2.44E+04 | 2.09E+04 | 1.28E+00 | 1.32E+00 |
600 | 5.59E+06 | 5.10E+06 | 1.11E+00 | 1.18E+00 |
800 | 8.87E+07 | 8.26E+07 | 1.06E+00 | 1.13E+00 |
1000 | 4.87E+08 | 4.64E+08 | 1.04E+00 | 1.10E+00 |
1200 | 1.57E+09 | 1.51E+09 | 1.02E+00 | 1.08E+00 |
1400 | 3.72E+09 | 3.61E+09 | 1.02E+00 | 1.07E+00 |
1600 | 7.22E+09 | 7.05E+09 | 1.02E+00 | 1.06E+00 |
1800 | 1.23E+10 | 1.20E+10 | 1.01E+00 | 1.05E+00 |
2000 | 1.90E+10 | 1.86E+10 | 1.01E+00 | 1.05E+00 |
(2) Reactions of Thermalized Carbonyl Oxides and Sulfur Dioxide. Landmark experimental work in the 1970's and 1980's provided evidence that a reliable measure of thermalized carbonyl oxide formed in an ozonolysis experiment was the amount of sulfuric acid aerosol formed (Scheme 1):
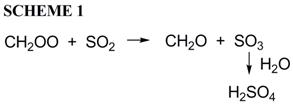
However, the only recent quantum chemical study of this system indicates that SO2 is capable of catalyzing the isomerization of carbonyl oxide 8 via a cyclic adduct 10 (Reaction 3):
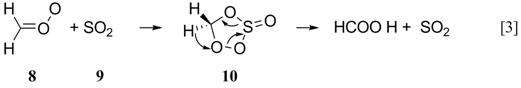
We have undertaken a comprehensive study of the reaction mechanism using the B3LYP/6-31+G(d,p) model chemistry. Our calculations have identified not only the five-membered ring intermediate 10, but also a four-membered ring (11) and an open diradical (12) (Scheme 4):
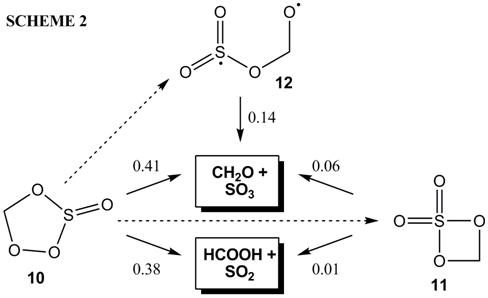
RRKM/Master equation simulations, based on the mechanism in Scheme 2 and B3LYP/6-31+G(d,p) quantum chemical data, predict the branching ratios shown next to each arrow. Around 60% of the SO2 reacting with CH2OO should be oxidized to SO3, while the other 40% drives the isomerization of CH2OO to the carboxylic acid. None of the intermediates is predicted to be collisionally stabilized under atmospheric conditions.