
ACS PRF | ACS
All e-Annual Reports

Safer, More Efficient Pipelines
Investigating the Mechanisms of Gas Hydrate Inhibition

Underwater flowlines are crucial components of the world’s energy infrastructure. However, they’re susceptible to blockages, creating severe safety and economic risks. Among the chief culprits of these blockages are gas hydrates, crystalline inclusion compounds comprised of hydrogen-bonded water cages (host) that can trap small guest molecules such as methane and ethane, eventually reducing the capacity or even blocking the flowline.
Traditional thermodynamic methods of suppressing hydrate formation are becoming prohibitively costly and environmentally undesirable. Therefore, alternative strategies need to be implemented to control hydrate formation within these hostile deepwater environments. With the support of the American Chemical Society’s Petroleum Research Fund, Drs. Carolyn Koh and Steven F. Dec are conducting research in this area.
Effective risk management of gas hydrate formation in deepwater environments requires time-dependent tools, including kinetic hydrate inhibitors and anti-agglomerants. Kinetic hydrate inhibitors delay hydrate nucleation/growth during the residence time of the fluid in the hydrate prone pipeline section(s). Conversely, anti-agglomerants allow hydrates to form, but prevent them from agglomerating so that the hydrate crystals can be transported as a slurry. These types of inhibitors are active at low concentrations of around 1 wt.% of the water content (compared to around 40-60 vol.% for thermodynamic inhibitors). The development of reliable and effective hydrate inhibitors requires advances in molecular-scale understanding of their effect on the kinetics and growth pathways of hydrate systems.
Koh and Dec’s team is examining the effect of model low-dosage inhibitors by applying molecular-level techniques, including in situ laser Raman spectroscopy and solid-state nuclear magnetic resonance (NMR) spectroscopy. At the same time, they’re studying the effect of gas hydrates’ structure and composition on the inhibition process.
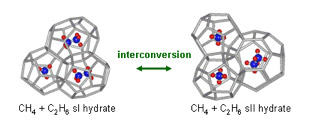
Other studies have used complementary high-pressure differential scanning calorimetric to examine these inhibition processes and the effect of inhibitors. The kinetic hydrate inhibitor, poly-N-vinylcaprolactam (PVCap), for example, was found to significantly change the metastability of sI and sII methane-ethane binary hydrates, inhibiting the formation of sII hydrate, while promoting the formation of sI hydrate.
The effect of kinetic hydrate inhibitors on crystal metastability, however, has not been previously examined. The team’s reformation experiments have demonstrated that samples containing PVCap gave significantly faster reformation rates than other approaches, indicating that the crystal structure of the hydrate is important in the effectiveness of the kinetic inhibitor. The results from this project have important implications to the petroleum industry and our fundamental understanding of the inhibitor-hydrate molecular interactions.
Back to top View Report