
ACS PRF | ACS
All e-Annual Reports
The Search for Better Catalysts
Gas-Phase Heterogeneous Catalysis on Metal Nanoparticles:
Size-Dependence

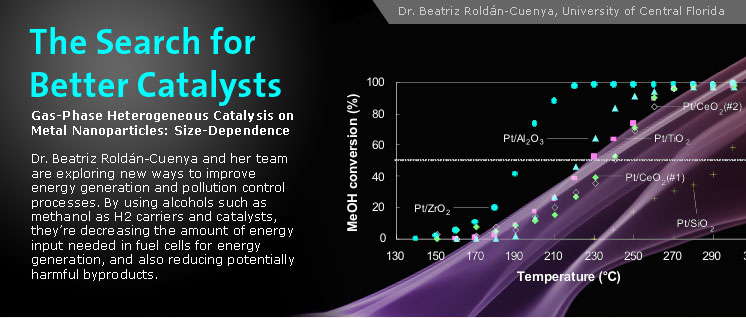
In recent years, economic, social, and environmental concerns have led to increased interest in alternative sources of energy that do not rely on fossil fuels. One of the most promising technologies in this area is the use of fuel cells for both stationary and mobile energy generation. Alcohols such as methanol (MeOH) have been proposed as potential H2 carriers, as they can be transported using current infrastructures and can be catalytically decomposed on-site to generate the necessary hydrogen gas to feed the fuel cells.
With the support of an award from the ACS-Petroleum Research Fund, Prof. Beatriz Roldán Cuenya and her research team at the University of Central Florida are focusing on the design and evaluation of novel nanoscale catalysts for methanol decomposition. “Our group is trying to improve the catalysts,” explains Roldán Cuenya, “that make it possible to decompose methanol and obtain hydrogen. We’re using platinum nanoparticles as catalysts, and evaluating the many factors that control the level of activity, as well as the selectivity, towards hydrogen production.”
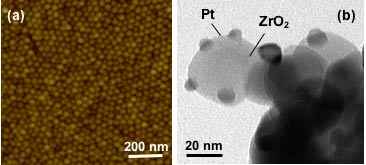
(a) Atomic force microscopy image of size-selected Pt nanoparticles deposited on SiO2/Si(111) [1]. (b) Transmission electron microscopy image of Pt nanoparticles supported on nanocrystalline ZrO2 [2].
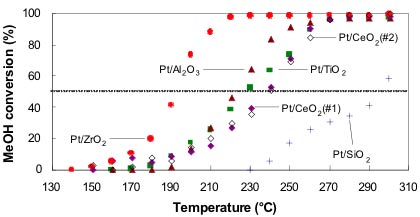
MeOH decomposition over Pt nanoparticles supported on nanocrystalline oxide powders: Pt/ZrO2 (full circles), Pt/Al2O3 (full triangles), Pt/TiO2 (full squares), Pt/CeO2(#1) (full diamonds), Pt/CeO2(#2) (open diamonds), Pt/SiO2 (crosses). The average nanoparticle sizes are: ~ 8-9 nm on TiO2, Al2O3 and ZrO2, and ~ 15-18 nm on SiO2 and CeO2 [2].
Finding results at the smallest level
Roldán Cuenya’s team is conducting basic investigations on the role of the nanoparticle size and nanoparticle-support interactions on their chemical reactivity and stability. The researchers synthesized size- and shape-selected platinum (Pt) nanoparticles (4, 6, and 8 nm) using the process of inverse micelle encapsulation, and deposited the nanoparticles on titanium dioxide (TiO2) [1]. They then used a packed-bed mass flow reactor and mass spectrometry to quantify the activity and selectivity of these catalysts for MeOH decomposition.
One area of particular interest for Roldán Cuenya’s group is in controlling the production of “poisoning” byproducts, which can form carbon deposits on top of the catalyst particles and prevent further reaction with methanol molecules. “Through our research,” Roldán Cuenya observes, “we’re working to improve the catalyst so that this effect is minimized, and we can produce mainly hydrogen, and not other unwanted, or hydrogen competitive, byproducts such as methane and other gases.”
Prof. Roldan Cuenya in front of her ultrahigh vacuum system used for the morphological, electronic and chemical characterization of nanoscale catalysts.
Among the catalysts tested, the smallest Pt nanoparticles (~4 nm) showed the best performance, including the lowest onset reaction temperature of ~145° C. For a common support (TiO2), the increase in activity with decreasing particle size is attributed to the high number of low-coordinated sites (steps and kinks) that appear, in increasing numbers, as the dimensions of the nanoparticles are reduced.

Undergraduate student Simon Mostafa (left) and graduate student Jason Croy in front of a bed-packed mass flow reactor dedicated to the investigation of the reactivity of supported metal nanoparticles at atmospheric pressure.
In a second study, the researchers deposited similarly-sized Pt nanoparticles on a series of reducible/non-reducible, nanocrystalline, metal-oxide supports (CeO2, TiO2, SiO2, ZrO2, and Al2O3) [2]. The nanoparticles deposited on ZrO2 were found to be cationic, and the most active for the decomposition of MeOH. The role of the support was found to be that of a stabilizer, a provider of preferential/additional sites of interaction, and a mediator among the different oxidation states of Pt.
The team’s work with catalysts could have implications for many areas of manufacturing, as well as energy generation and pollution control. “We expect,” says Roldán Cuenya, “that advances in this field may have widespread positive consequences by decreasing the energy input required as well as the yield of potentially harmful byproducts associated with chemical synthesis processes.”
[1] J. Croy et al., Catal. Lett. 118, 1 (2007). [2] J. R. Croy, S. Mostafa, J. Liu, Yongho Sohn, H. Heinrich, B. Roldán Cuenya, Catal. Lett. 119, 209 (2007). Back to top