
ACS PRF | ACS
All e-Annual Reports

A More Efficient Way to Refine Energy
Mesh-Adjustable Molecular Sieves for General Use in Gas Separation
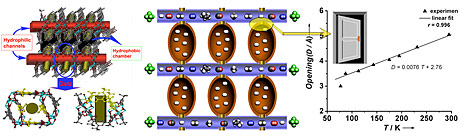
Illustration provided by Dr. Jian-Rong Li of the Zhou Research Group in the Department of Chemistry, Texas A&M University
Gas separation is an essential industrial process in the energy industry, and rising energy costs are making it increasingly important to find more efficient methods for doing so
.One of the main challenges in the process is that the gas molecules that need to be separated are normally very similar in size and physical properties. Zeolite molecular sieves, when available, are very efficient in size-exclusion separation.
However, when the size disparity of the two gases that need to be separated is very small, a molecule sieve with the precise mesh size isn’t always available. In such cases, a mesh-adjustable molecular sieve (MAMS) that can always meet the separation needs is highly desirable.
Creating a next-generation sieve
When zeolite molecular sieves are used in gas separation, it is generally difficult to ‘tune’ the pore size and the surface properties of the pore wall. Conversely, in metal-organic frameworks (MOFs), not only the pore size but also the surface properties can be tuned systematically. MOFs are zeolite-like materials containing metal clusters linked by organic ligands, and occupy a unique place at the intersection of inorganic chemistry and materials science.
Recently, chemists from Texas A&M University have synthesized a MOF-based mesh-adjustable molecular sieve, MAMS-1. Led by Professor H.C. Zhou, Ph.D., and with the support of an award from the ACS Petroleum Research Fund, the team is investigating a unique, adjustable mesh sieve. The material has a graphitic framework structure, in which atoms are connected covalently in each layer, but are held together by weak interactions between adjacent layers.
“We believe our work has particular potential to improve energy-related gas separation,” explains Dr. Zhou. “For example, natural gas reserves are always contaminated by CO2 and nitrogen. In terms of molecular size, methane, the main component of natural gas, and these two contaminants are extremely close, making it very challenging and expensive to purify natural gas. We’re making progress on finding more energy-efficient ways to do so.”
Using temperature for fine-tuning
The mesh size of the framework is linearly related to temperature. The mesh range falls between 2.9 and 5.0 Å, covering the size range of almost all commercially important gas separations. In principle, by precise temperature control, any mesh size within this range can be achieved. MAMS-1 shows selective gas adsorption of H2 over CO/O2/N2 at 77 K, O2 over CO/N2 at 87 K, N2 over CO/CH4 at 113 K, CH4 over C2H4 at 143 K, C2H4 over C3H6 at 195 K, and C3H6 over iso-C4H10 at 241 K.
Ongoing efforts in the Zhou Research Group focus on changing organic ligands to systematically adjust the structure and adsorption properties of MAMSs for gas separation at ambient temperatures and applicable pressures. “Using this technology,” Zhou explains, “one can potentially separate almost any two gases with any size differences.”
Back to top