ACS PRF | ACS
All e-Annual Reports

45134-B7
Competition between Mesogens in Pyridone-Based Supramolecular Calamitic, Banana, and Discotic Liquid Crystals
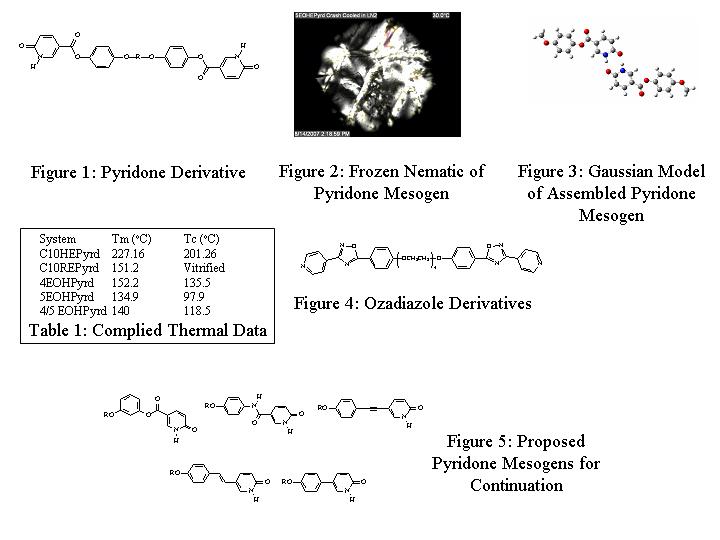
In the first year, the synthesis of pyridone-based esters ws undertaken. It was found that while the substitution chemistry of pyridones was difficult, the species were capable of reacting through nucleophilic acyl substitutions. Metal-based coupling reactions (such as Suzuki couplings) proved problematic, as the pyridine functionality seemed to chelate the metal from the catalyst, rendering the reaction inert. These results provided the groundwork for the rest of the project. In the second year, a series of bis-functionalized pyridone terminated esters were synthesized. One set of supramolecular esters, seen in Figure 1, is based on alkylated hydroquinone derivatives. The R group in these molecules included a decane chain, as well as a series of oligomeric (3,4,5) ethyleneoxy chains. Supramolecular polymers produced flexible, long-lived fibers pulled from the melt, but interestingly produced only a frustrated nematic phase observable only upon crash cooling the isotropic melt in liquid nitrogen. It is believed that the nematic phase could only be captured in such a dramatic fashion because the overall structure of the assembled pyridone species would be too irregular to effectively form a mesogenic phase. Figure 2 shows the frozen nematic phase obtained from one of these complexes, and Figure 3 displays the irregular hydrogen bonding pattern in pyridone-type assemblies. Table 1 shows all of the observed thermal transitions. Results of this work were presented at the Boston ACS meeting in August of 2007. A manuscript is in development to be submitted to Liquid Crystals. This paper will have three undergraduate authors. Other work funded by this grant included a side-project investigating the liquid crystalline properties of polymeric oxadiazoles. A series of bis-pyridyl-terminated oxadiazole molecules was synthesized. A sample structure is seen in Figure 4. These molecules were then used to generate supramolecular liquid crystalline polymers when hydrogen bonded to bis-acids with similar flexible spacer groups. It was interesting to note that very few of the complexes generated produced any mesogenicity. It was believed that the irregular (bent) structure once again hindered the formation of the liquid crystalline phase. This is especially interesting, as both this and the pyridone systems produce strongly liquid crystalline small molecules. A study of the effects of molecular “kinks” and polarity is currently underway using GAUSSIAN molecular modeling techniques and synthetic methodologies. Resorcinol-based esters (Figure 5) will be synthesized in order to vary the angle of the association structure and determine the effect on mesogenicity. Amide species will also be synthesized to determine if the lateral hydrogen bonding of the amides can help drive mesophase formation and overcome the irregularity introduced by the “kink” of the structure. Additionally, metal coupling reactions will be carried out using 2-benzyloxy pyridine derivatives in order to increase the mesogen length without introducing a “kink” in the structure using alkenes, alkynes and aromatic species. The benzyl protecting group will then be cleaved through hydrogenation to produce the pyridone. The ability of the species to form discotic and banana-shaped mesogens will also be investigated.