www.acsprf.org
Reports: AC448587-AC4: 'Radically' Green Approaches To Hydrocarbon Functionalization Via Allyl Transfer
James M. Tanko , Virginia Polytechnic Institute and State University
Introduction
Hydrocarbon functionalization via an allyl transfer reaction (Scheme 1, X = Br) using various allyl bromide substrates, has been studied in our group1. In work by the ACS-PRF, we found that replacement of Br by phthalimido-N-oxyl (PINO) was successful, and has helped make this chemistry environmentally benign2. Reactions of allyl-phthalimido-N-oxyl (PINO) compounds for hydrocarbon functionalization have yielded excellent results using high temperature initiators (di-tert-butylperoxide) and these reactions are now under investigation with respect to low temperature initiators (triethylborane, di-tert-bytylhyponitrite)3. Similarly, functionalization of ethers (tetrahydrofuran, 2-methyltetrahydrofuran, dioxane, diethyl ether etc.) has shown interesting results and is being studied further.
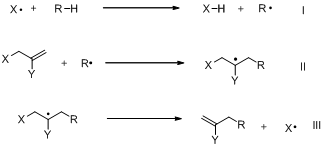
Scheme 1. Propagation steps of allyl transfer reactions
The advantage of PINO chemistry is the byproduct of the reaction, NHPI (N-hydroxyphthalimide), is easily recovered and separated as a while solid at the end of the reaction. We synthesized various “allyl-PINO” substrates using several published methods. Although, we could not completely eliminate the use of allyl bromides from these synthetic pathways, our preliminary objective is to study PINO chemistry in hydrocarbon functionalization to establish feasibility, and then focus on an improved synthesis of “allyl-PINO” substrates afterward.
The allyl-PINO chemistry works well with DTBPO (di-tert-butylperoxide, a convenient initiator that generates t-butoxyl radical), resulting in excellent yields. We compared the effect of leaving group (X = Br vs. PINO), concluded that the reactions are cleaner with allyl-PINO substrates (Scheme 2, Table 1). Mass balances are higher in case of PINO although yields are higher in case of Br (Table 1). A comparison of the kinetic chain length suggests that β-fragmentation could be the problem step in case of PINO (Scheme 3), because chain lengths of bromide substrates are almost 5X the chain lengths of PINO substrates (Table 2).
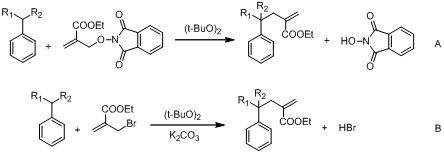
Scheme 2. Hydrocarbon functionalization using A) allyl-Br and B) allyl-PINO substrates
|
Table 1. Allyl-Br vs. allyl-PINO substrates in the allyl transfer reaction |
||||||
|
allyl-Br |
allyl-PINO |
|||||
|
|
% Starting Material Left |
% Yield |
% Mass balance |
% Starting Material Left |
% Yield |
% Mass Balance |
|
R1=R2=Ha |
- |
35 |
35 |
21 |
48 |
69 |
|
R1=H, R2=CH3b |
- |
70 |
70 |
44 |
56 |
100 |
|
R1=R2=CH3b |
- |
36 |
77 |
75 |
20 |
95 |
a Reaction time = 42 h, b Reaction time = 3 h. All reactions were performed in neat toluene, ethyl benzene or cumene, using 20 mol % di-tert butylperoxide as an initiator at 120 oC
|
Table 2. Kinetic chain length comparisons between allyl-Br and allyl-PINO substrates in the allyl transfer reaction. |
|||
|
Initial Chain Lengths |
|||
|
PINO |
R1=R2=H |
R1=H, R2=CH3 |
R1=R2=CH3 |
|
266 |
129 |
11.5 |
|
|
Br |
800 |
N/D |
60 |
Lewis acid catalyzed allyl transfer reactions
In our previous annual report, we noted that the use triethylborane (TEB)/O2 as an initiator5 in allyl transfer rections of allyl-Br and allyl-PINO substrates did not result in significant product formation (<40 % yield). More recently, we decided to use a Lewis acid (Scheme 3) in order to make the allyl substrate more electrophilic, hopefully facilitating the addition step. Addition of nucleophilic radicals to olefins are known to be facilitated by the addition of a suitable Lewis acid.6 In our work, we found that product yields improved dramatically. The results are summarized in Table 3. Ongoing laser flash photolysis studies will further investigate more about the role of Lewis acid in ally transfer reaction by determining the rate constants the addition/elimination sequence in the presence and absence of a Lewis acid.
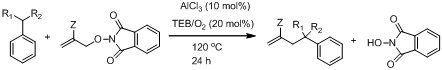
Scheme 3 Hydrocarbon functionalization using Lewis acid (AlCl3)
|
Table 3. Results for hydrocarbon functionalization in the presence of a Lewis acid (AlCl3)
|
|||||
|
Entry |
R1 |
R2 |
Z |
Yield (%) |
Mass balance (%) |
|
1 |
H |
H |
Ph |
68 |
68 |
|
2 |
CH3 |
H |
Ph |
89 |
89 |
|
3 |
CH3 |
CH3 |
Ph |
72 |
87 |
|
4 |
H |
H |
COOEt |
62 |
72 |
|
5 |
CH3 |
H |
COOEt |
80 |
80 |
|
6 |
CH3 |
CH3 |
COOEt |
70 |
82 |
In all the above reactions, solvent was hydrocarbon and 20 mol % di-tert butyl hyponitrite was used as initiator.
Ether functionalization via allyl transfer reactions
Functionalization of C-H bond of ether is difficult because of its low reactivity7. Most of the existing methods involve the use of metals or organometallic reagents. Because ethers like THF, Me-THF, dioxane, etc. posses weak C-H bonds, and more or less similar BDEs to toluene or cumene, our idea was to treat them as we did hydrocarbon and carry out allyl transfer reactions (Scheme 4 and 5). We successfully reported ether functionalization via allyl transfer reaction (Table 4 and 5).

Scheme 4. Ether functionalization (Z= COOEt) via allyl transfer using di-tert butyl peroxide (DTBPO)
|
Table 4. Results for ether functionalization (Z=COOEt) via the allyl transfer reaction |
|||
|
Ether |
Z |
Yield |
Starting Material Left |
|
THF |
COOEt |
85 |
- |
|
2-MeTHF |
COOEt |
62 |
- |
|
Diethyl ether |
COOEt |
45 |
35 |
|
1,3-dioxane |
COOEt |
56 |
- |
|
1,4-dioxane |
COOEt |
75 |
10 |
In all the above reactions, the ether was the solvent and 20 mol % di-tert butyl peroxide was used.

Scheme 5. Ether functionalization (Z= Ph) via allyl transfer using di-tert butyl peroxide (DTBPO)
|
Table 5. Results for ether functionalization (Z=Ph) via the allyl transfer reaction |
|||
|
Ether |
Z |
Yield |
Starting Material Left |
|
THF |
Ph |
83 |
10 |
|
2-MeTHF |
Ph |
66 |
15 |
|
Diethyl ether |
Ph |
29 |
30 |
|
1,3-dioxane |
Ph |
|
- |
|
1,4-dioxane |
Ph |
63 |
15 |
In all the above reactions, solvent was ether and 20 mol % di-tert butyl peroxide was used.
In efforts to reduce the concentration of ethers in the reaction to stoichiometric (rather than solvent) amounts, we performed a series of experiments with varying concentrations of ether in acetonitrile solution (Scheme 6) to determine the best minimum substrate concentration that did not compromise reaction yields. We that only 3 equivalents of ether were needed when the allyl transfer reaction was conducted in acetonitrile solvent (Table 6).
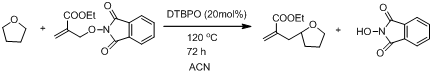
Scheme 6 C-H functionalization of THF
|
Table 6. Results of experiments to find the optimal concentration of ether substrate in the allyl transfer functionalization of ethers |
|
|
THF (Equiv) |
Yield % |
|
1 |
62 |
|
2 |
75 |
|
3 |
83 |
|
4 |
85 |
Synthesis of a benzyl radical precursor for laser flash photolysis
We have recently synthesized the benzyl radical precursor8 (Scheme 7) for laser flash photolysis studies designed to measure the absolute rate constants for the addition/elimination step of the allyl transfer reaction. Compound 7a will be used with allyl-PINO substrates to calculate the rate constants for benzyl radical additions to olefins.
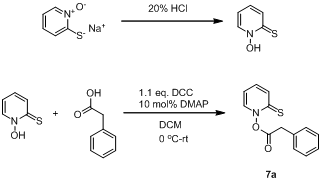
Scheme 7 Synthetic scheme to yield 7a (Benzyl precursor)
Research Plans- Amine functionalization
In the next year, we plan on extending this research to C-H functionalization of Amines9 (Scheme 8)
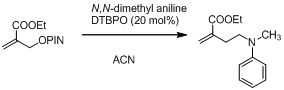
Scheme 8 Amine functionalization via allyl transfer.
References:
1. Struss, J. A.; Sadeghipour, M.; Tanko, J. M., Radical additions to allyl bromides. A synthetically useful, [`]Tin-Free' method for carbon-carbon bond formation. Tetrahedron Lett. 2009, 50 (18), 2119-2120.
2. Shraddha Patil; Liang Chen; Justin Custiss; Tanko, J. M., Radically” green approaches to hydrocarbon functionalization via allyl transfer using phthalimido-N-oxyl radical (PINO). Org. Biomol. Chem. 2011, Submitted.
3. Protasiewicz, J.; Mendenhall, G. D., Di-tert-butyl hyponitrite as a source of alkoxyl radicals for dimerization. J. Org. Chem 1985, 50 (17), 3220-3222.
4. (a) Pines, H.; Alul, H.; Kolobielski, M., Notes - Bromination of α-Methylstyrene with N-Bromosuccinimide. J. Org. Chem 1957, 22 (9), 1113-1114; (b) J Villieras; Rambaud, M., Synthesis 1982, 924-926; (c) D Albert; Celine, T., Synthesis 2006, 10, 1635-1638.
5. Miyabe, H.; Yamaoka, Y.; Takemoto, Y., Triethylborane-Induced Intermolecular Radical Addition to Ketimines. J. Org. Chem 2005, 70 (8), 3324-3327.
6. Sibi, M. P.; Porter, N. A., Enantioselective Free Radical Reactions. Acc. Chem. Res. 1998, 32 (2), 163-171.
7. Lee, S. Y.; Lai, T. H.; Choi, K. S.; Chan, K. S., Room-Temperature Selective Aliphatic CarbonCarbon Bond Activation and Functionalization of Ethers by Rhodium(II) Porphyrin. Organometallics 2011, 30 (14), 3691-3693.
8. Aveline, B. M.; Kochevar, I. E.; Redmond, R. W., Photochemistry of N-Hydroxypyridine-2-thione Derivatives: Involvement of the 2-Pyridylthiyl Radical in the Radical Chain Reaction Mechanism. J. Am. Chem. Soc. 1995, 117 (38), 9699-9708.
9. Godula, K.; Sames, D., C-H Bond Functionalization in Complex Organic Synthesis. Science 2006, 312 (5770), 67-72.
