www.acsprf.org
Reports: DNI149181-DNI1: New Catalytic Asymmetric Reactions of Allylsilanes and Isatins
Annaliese K. Franz, PhD , University of California (Davis)
Enantioselective catalysis is the most efficient method toconstruct the carbon-carbon bonds of stereochemically-defined molecules fromsimple feedstock chemicals. Challenges remain to design and develop efficientcatalysts with low catalyst loading, provide synthetic methods that acheivehigh enantioselectivity, incorporate polar groups and heterocycles, anddetermine the effects of additives and counterions. The goal of this researchis to develop new catalytic asymmetric reactions of allylsilanes and isatinsusing the reactivity of chiral acid complexes with chelating electrophiles forthe efficient synthesis of complex heterocycles from simple feedstockchemicals. Our research has focused on hydoxy-oxindoles and spirocyclicoxindoles because they are attractive targets for synthetic organic researchdue to their biological activity and occurrence as the core structure innatural products.
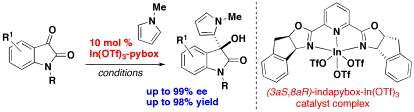
In the previous granting period, wehave compared the activity and selectivity of diverse Lewis acid catalysts andshown that chiral scandium(III)- and indium(III)-pybox complexes as efficientcatalysts for the direct enantioselective addition of a broad scope ofnucleophiles to isatin electrophiles. We described the first enantioselectiveaddition of indoles to isatins, which proceeds under mild conditions using aSc(III)-pybox complex with as low as 1 mol% catalyst loading. The optimalenantioselectivity was observed using a 2,6-bis[(3aS,8aR)-3a,8a-dihydro-8H-indeno[1,2-d]oxazolin-2-yl]pyridine(i.e. indapybox) ligand. Aspart of this investigation, the reactivity and selectivity ofindium(III)-indapybox complexes were compared and also shown to be effective aschiral catalysts for Friedel-Crafts reactions with isatins. We also discovereda novel spirocyclization reaction with oxazole nuclophiles for thestereoselective synthesis of spirocyclic oxazolines. Here the choice of metalcatalyst is important for the diastereoselectivity, while the structure of theoxazole determines the regiochemistry of the oxazoline.
In this granting period, we havecontinued to investigate enantioselecitve reactions of isatin electrophiles,leading us to compare new catalyst conditions and demonstrate theenantioselective synthesis of silyl spirooxindoles and spiroindolones. We areparticularly interested in allylsilanes, which are readily available,non-toxic, and versatile reagents for organic synthesis. Although fairly weaknucleophiles, allylsilanes exhibit a dynamic reactivity pattern that isdependent on the electronic and steric properties of the silyl group, the electrophilicpartner, and the reaction conditions. Upon optimization of the scandium complexcatalyst utilized for the indole addition, we have developed methodology forefficient reactions of allylsilanes with isatins.
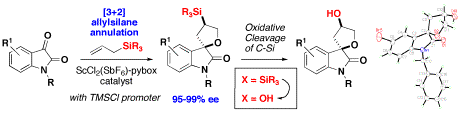
We have demonstrated the firstexample of an asymmetric catalytic [3+2] allylsilane annulation reaction,providing the synthesis of silyl- and hydroxy-spirooxindoles with superbenantioselectivity. The allylsilane annulation reaction represents a powerfulmethod for efficient stereoselective synthesis of complex heterocycles andcarbocycles; however, this reaction represents a particular challenge becausecatalysts often favor the competing allylation pathway. We have shown that a chiral cationicScCl2(SbF6)pybox complex with TMSCl as an essentialactivator provides an efficient system for this reaction to proceed withconsistently high enantioselectivity at room temperature. The counterion andhalide ligands on the scandium complex play an essential role to controlpathway selectivity for the formation of the annulation product while theindapybox ligand effectively controls the enantioselectivity, For greatest syntheticutility, we utilize a benzhydryl silyl group, which is bulky enough to suppressthe elimination pathway, while also containing a "removable" aromatic moietythat can be converted to a hydroxyl group under mild oxidation conditions.Using the benzhydryl allylsilane, the annulation reaction retains excellentenantioselectivity, and the high enantiomeric excess is preserved uponoxidation of the Si-C bond.
We have also expanded the scopeof heteroaromatic nucleophiles and have developed the first asymmetriccatalytic method for pyrrole additions to isatins. The choice of chiral metal complexproved to be important for yield and selectivity. The indium(III)-pybox catalyst provides sufficient catalyticactivation of isatins, while also controlling the regio- and enantioselectivityfor the addition. High enantioselectivity is maintained with the In(OTf)3-pyboxcomplex under a variety of conditions for both NH and N-alkylated isatins. Itwas also observed that interactions with a 4-chloro substituent hinder thepyrrole addition in this system, which is notable because this representats acase of divergent reactivity for indoles and pyrroles. The success of theindole addition confirms that the effect is not based on an interaction of themetal-ligand complex with the 4-chloro substituent, but rather that thedistinction in reactivity is attributed solely to the nucleophilic species(i.e. pyrrole vs indole). The effect of a counterion was also investigated forthis reaction and the addition of NaSbF6 was shown to improvecatalytic activity. The additionof NaSbF6 likely generates a more cationic catalyst complex thatexhibits increased catalytic activity. 19F NMR studies for theIn(OTf)3-pybox indicate that the triflate ligands are partially ortotally dissociated for the metal-ligand complex.
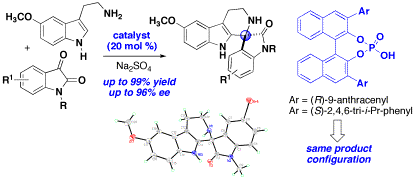
We have also developed an enantioselective Pictet-Spenglerreaction between isatins and 5-methoxy tryptamine catalyzed by chiralphosphoric acids, providing spiroindolones in excellent yields andenantioselectivity (up to 96% ee). Spirooxindole tetrahydro-b-carboline products of this typehave recently been identified as a potential treatment for Malaria based onin-vivo activity. We initially screened various chiral Lewis acid complexes,such as scandium(III)-indapybox, and while these Lewis acids were effective tocatalyze the spirocyclization, the chiral complexes did not induceasymmetry. Further evaluationdemonstrated that spirocyclization was effectively catalyzed usingBINOL-derived phosphoric acid catalysts. A comparison of catalysts provides insight for the substrate scope andfactors responsible for efficient catalytic activity and selectivity in thespirocyclization. It was particularly notable that chiral phosphoric acids withdifferent 3,3'-substitution on the binaphthyl system and opposite axialchirality afford the spiroindolone product with the same absoluteconfiguration.
These synthetic and catalyst studies contribute to a generalunderstanding of activation and selectivity for chiral acid complexes withchelating electrophiles. This funding has also contributed to the training of 4graduate students and 3 undergraduate students. Of these students, one student applied for and received anNSF graduate research fellowship. 3 students and the PI have presented research at ACS National meetings.Three publications have resulted from this work, and two additionalpublications have been accepted and are in press (in Org. Lett. and Angew. Chem., Int. Ed.).