www.acsprf.org
Reports: DNI1050390-DNI10: Control of Interpenetration in Metal-Organic Frameworks via Spatial Protecting Groups
Ognjen S. Miljanic, PhD , University of Houston
Introduction and Objectives
Metal-organic frameworks (MOFs) are porous crystalline materials created by connecting metal cluster "nodes" through oligofunctional organic "struts" into an infinite framework. High thermal stability, easy synthetic modification, and high porosity have made MOFs promising candidates for applications in gas storage and separation, catalysis, and drug delivery. Pore sizes in MOFs can be enlarged by increasing the separation between metal nodes, but this method fails with very long linkers, as Nature fills up the empty space through interpenetration—creation of one or more identical copies of the framework within the voids of the original net. The original objective of our work is to circumvent interpenetration in MOFs using spatial protecting groups (SPGs): bulky functionalities that would be attached to the organic struts, making them appear bulkier—and less prone to interpenetration—during MOF synthesis. SPGs would be removed postsynthetically to reveal large pores that could not have been introduced directly. Our interests broadened to also include the study of encapsulation of small molecules within MOF pores. If selective, such encapsulation can lead to spontaneous ordering (self-sorting) phenomena, analogous to the compartmentalization in living systems.
Results
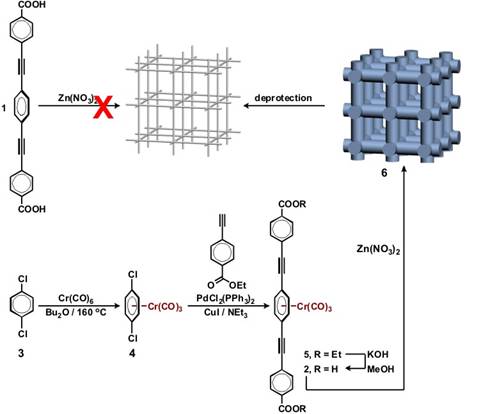
Our strategy for avoiding interpenetration in MOFs is illustrated in Figure 1. Long linkers such as 1 form interpenetrated networks with small pore sizes. We speculated that if Cr(CO)3 (chromium tricarbonyl) moieties were coordinated to the central benzene ring of 1, this new linker 2 would "sweep" much larger volume than 1, thus minimizing the entropic incentive for interpenetration. Linker 2 was synthesized by a Sonogashira coupling of chromium-coordinated 1,4-dichlorobenzene (3) with alkyne 4. Saponification of 5 produced acid 2, which we attempted to coordinate to zinc centers in order to produce spatially protected MOF 6. While infrared spectroscopy suggests that this reaction proceeds as planned, we have so far not been able to produce single crystalline materials; we interpret this failure as a consequence of partial oxidation and decomplexation of chromium pendants—which then precludes the production of materials with long-range order. Our current efforts are directed at rigorous exclusion of air from the MOF solution samples in an inert-atmosphere glovebox. Once these conditions are optimized and single crystalline spatially protected MOFs are prepared, decomplexation of chromium will be achieved using controlled diffusion of vapors of an oxidant (e.g. iodine) into crystals of MOF 6.
In parallel to our work on the oxidative deprotection of SPGs, Telfer's and Cohen's groups disclosed independent strategies for the postsynthetic revelation of empty space within a MOF. The former team used thermolysis of bulky -NHBoc groups to reveal -NH2 functionalities, while Cohen photochemically cleaved bulky nitrophenylesters to catechols. These methods are complementary to ours, since it would be desirable to have a broad arsenal of potential deprotection conditions.
Figure 1. Our strategy for avoidance of interpenetration includes initial creation of a MOF with bulky ligands, followed by subsequent cleavage of these spatial protecting groups.
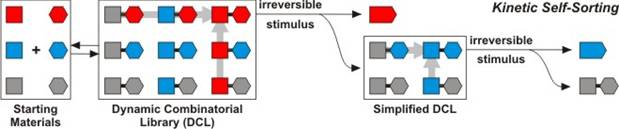
The second avenue of our research studies compartmentalization processes, both in the classical heterogeneous sense (physical compartmentalization), as well as in the sense of chemical isolation and non-interference (chemical pseudo-compartmentalization). We are interested in examining the influence that high-barrier MOF encapsulation and other irreversible stimuli exert over complex mixtures of equilibrating compounds. As the model system, equilibrating mixtures of imines were chosen; imines have the general molecular formula X-CH=N-Y and, when multiple imines are present in the same solution, they readily switch their substituents. Such equilibrating Dynamic Combinatorial Libraries (DCLs) respond to external stimuli by amplifying the component that adopts best to those stimuli. Our work has demonstrated that very complex mixture of imines will spontaneously simplify (self-sort, Figure 2) when exposed to an irreversible stimulus that shows selectivity in removal for certain components of the mixture. Irreversible removal of the most reactive component of the mixture sets into motion a sequence of events mandated by the Le Chatelier principle. As illustrated in Figure 2, within the equilibrating DCL, one species will best respond to the irreversible stimulus—in this example, that would be the "red"-"red" combination. Removal of that species through an irreversible process will cause the mixture to re-equilibrate and generate more of the just-removed component. Eventually, removal of "red"-"red" combination will extract "red" components out of all the cross-combinations, thus both simplifying the mixture and leading to exclusive high-yield production of this fast-reacting species. In the next step of the process, a different stimulus can be applied, which will now express the "blue"-"blue" combination at the expense of all crossover members. Ultimately, mixture with n2 components is simplified into just n components in a process that is controlled by both thermodynamic and kinetic factors. We have demonstrated that this self-sorting operates under chemical conditions (i.e. slow irreversible oxidation), as well as under the influence of irreversible physical stimuli, such as distillation. Both of these methods were recently published. Very recently, we have also shown that high-barrier encapsulation within crystals of MOFs with different pore sizes can also be used to self-sort a mixture of equilibrating imines.
Figure 2. Schematic representation of kinetically controlled self-sorting processes which were discovered during the course of the PRF-funded project.
Impact on the Careers of the PI and Students Involved in the Project
Based on the support from the PRF, the PI has published four out of his group's first five articles. For these accomplishments, PI was recognized by the 2011 Thieme Chemistry Journal Award and the 2010 Younger Chemist of the Year Award, given by the Greater Houston Section of the American Chemical Society, and invitations to present fifteen talks at universities and conferences. Karolina Osowska (postdoc) co-authored three of the four articles published with the PRF support and has started a job as a researcher in the Polish biotechnology company Selvita. Teng-Hao Chen (graduate student), who was directly supported by the PRF funds, is currently preparing his first publication and will advance to PhD candidacy this Fall; his poster, presented at the Spring 2010 meeting on the American Chemical Society, was selected for the SciMix event.