www.acsprf.org
Reports: UNI150466-UNI1: Novel Nucleophilic Trapping Reactions of Carbocations in Ionic Liquids
Elizabeth D. Kochly, PhD , Mills College
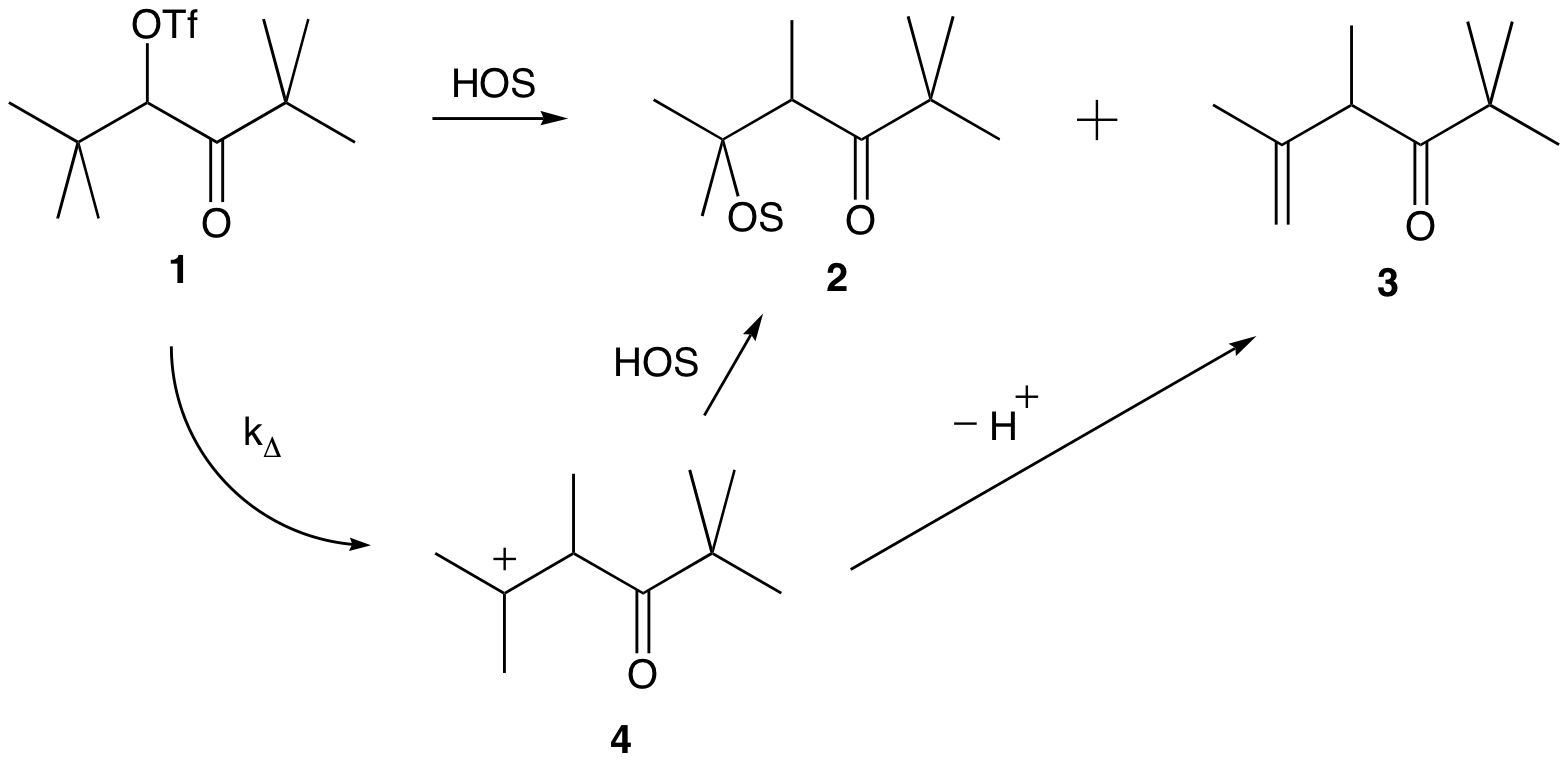
The main goals for our research are three-fold: 1) to expand our fundamental knowledge of carbocations, ionic liquids and the interactions between the two, 2) to synthesize and study novel organic compounds, and 3) to provide undergraduate women science students at Mills College an opportunity to perform directed research. Our current project is an investigation of the effect of ionic liquids on solute nucleophilicity. We are studying the solvolysis of pivaloyl triflate in binary solvent systems of ionic liquid and various organic alcohols. It is well known that pivaloyl triflate 1 solvolyzes via a kD process to yield rearranged products 2 and 3 (scheme 1). Upon formation of the rearranged carbocation, 4, the solute acts either as a nucleophile or a base forming two potential products: substitution and elimination. We wished to determine whether the presence of ionic liquid would affect the product ratio.
Scheme 1. Solvolysis of pivaloyl triflate in a protic solvent.
The solvolysis reaction was carried out in solvent mixtures of bmimNTf2 with a variety of organic alcohols: methanol-d4, ethanol-d6, trifluoroethanol-d3 and acetic acid-d4. The concentration of alcohol was varied from 5% to 100% (by weight). Product ratios were determined by NMR integration. It was found in all cases that as the percentage of alcohol co-solvent was decreased, more elimination product was observed. Or, stated differently, more ionic liquid led to more elimination product. This effect is more pronounced at very low concentrations of alcohol. Our studies suggest that the alcohol co-solvent becomes more basic (rather than more nucleophilic) in the presence of ionic liquid. This would appear to challenge the claim that ionic liquids increase the nucleophilicity of solutes. Studies are ongoing.
A primarily undergraduate institution, Mills College is a women's college with a substantial population of women of color. Funding from the ACS PRF has given some of these women valuable opportunities to participate in scientific research. Specifically, three students had the opportunity to work on these projects. Two of these students have now graduated. One earned a BS in chemistry and is applying to graduate school. The other is in a Masters of Public Health program at UCSF. The third student is now a senior completing her BS in Chemistry. All three have benefited greatly from working on these projects. This practical, hands-on experience has given these students valuable skills that will help to prepare them for their future studies.
The principal investigator has also greatly benefitted from ACS PRF support. As a pre-tenure faculty member, this funding has allowed her to get her research up and running. She is currently writing up the results from this project as a preliminary communication.