www.acsprf.org
Reports: B548436-B5: Interaction of Polyoxometallates with Organically-Modified Silica
Michael A. Everest, PhD , Westmont College
During the third year of the grant period, significant progress was made in making reproducible gradients of 3-aminopropyltrialkoxysilane on fused silica substrates. As has been mentioned in previous reports, much effort has gone into fine-tuning the surface preparation procedure. This work has continued. Specifically, we are now treating the surfaces with an oxidizing acid (piranha solution) before exposing to 3-aminopropyltriethoxysilane. Moreover, we are also annealing the substrates in an oven following vapor deposition. These two steps together have resulted in significant improvement in surface coverage as indicated by contact angles (determined with capital equipment procured in year one of the grant period).
Figure 1 shows the contact angle of a small drop of water at several positions on the prepared substrate. Contact angles for three different surface exposure times are shown. For 55 s deposition time, only the edge of the substrate closest to the vapor source has been coated with a monolayer. At long exposure times of 90 s, the entire surface has been coated with a monolayer. The optimal surface gradient is achieved after 80 s exposure times after which a distinct gradient is obtained in approximately the middle of the substrate. Preparations such as this will be the focus of ongoing work with evanescent-wave cavity ring-down spectroscopy.
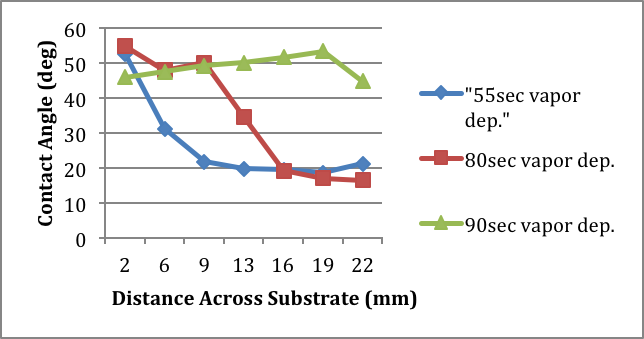 |
| Figure 1 Contact angle of water on a fused-silica surface exposed to 3-aminopropyltriethoxysilane for various amounts of time. |
Also during the past year, we have begun attempts to prepare surfaces modified with negatively charged substrates. Preliminary experiments have been conducted on the formation of gradients with thiol-terminated silanes that we then hope to oxidize to the corresponding sulfonic acid. We have indirect evidence that we have successfully made a sulfonic-acid modified fused-silica surface, but further work is needed to demonstrate this unambiguously. Cavity Ring-down Imaging of a Surface
During the past year, we greatly improved our ability to use evanescent-wave cavity ring-down measurements for imaging a surface. Our preliminary data that were included in the original grant proposal are included in Figure 2. The resolution was poor for two reasons: (1) large steps taken with the stepper motors holding the substrate, and (2) the inherent beam width of the laser. We have improved both of these factors. Decreasing the step size is a simple matter of settings in the software used for data acquisition. However, using a step size smaller than the laser beam width is of little use and would still result in poorly resolved images. The laser beam width in the cavity is decreased by changing the cavity length. Changing the cavity length from 95 cm to 9.5 cm will result in decreasing the beam waist from 0.25 mm to about 0.15 mm. Figure 3 shows an improved image of a spot on a fused-silica substrate. The image is 50x50 pixels, and the cavity length is 9.5 cm. The sample in Figure 3 is a drop of a dilute solution of 3-aminopropyltriethoxysilane in toluene that has been allowed to evaporate. Combining the new spatial resolution with chemical gradients discussed above should open new doors to gradient-based studies of various molecules with chemically modified surfaces.
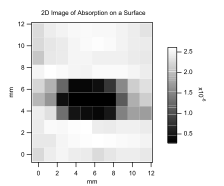 | 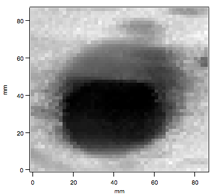 |
| Figure 2 Preliminary data for evanescent-wave cavity ring-down imaging. | Figure 3 High-resolution evanescent-wave cavity ring-down imaging. |
During the past year, the PI has successfully applied for a position at another primarily undergraduate institution. He has moved from George Fox University (Newberg, OR) to Westmont College (Santa Barbara, CA). During the application and interview process, it was clear that having an active PRF Type B grant was a significant factor in the eyes of the hiring committee. Westmont College offered enough startup funding to buy all the equipment in the PI's laboratory, so the research program will continue uninterrupted. Moreover, Westmont College requires all chemistry majors to participate in research for two semesters. These students do not need the financial support of the grant, but they will greatly benefit from the ongoing work and the equipment and supplies made available by the grant funds. Impact on Supported Students
The two students supported during year one of the grant graduated from George Fox University in May of 2011. One was given the departmental Outstanding Major of the Year award from the Department of Chemistry. She applied and was accepted to several Chemistry PhD programs including University of California, Berkeley, one of the most highly ranked programs in the nation. The other student from the first year got a job immediately upon graduation working in a chemistry-related field (water quality testing). The research these students were able to participate in with support from the PRF was certainly a factor in all that these students have attained.The students supported during year three of the grant (no students were supported during year two owing to the PI being on sabbatical) are currently juniors at George Fox University. Largely because of his experience in research funded by the PRF, one of the students has decided to pursue graduate work in chemistry instead of (or in addition to) his previous plan of only practicing medicine. Current Status of Activities
The PI was on sabbatical during the second year of the grant, therefore there remains approximately one-year of funding that has not been spent. A one-year no-cost extension will be requested, and the balance of the funds will support the PI and two undergraduate students during the summer of 2012 at Westmont College. We will continue the work discussed above, measuring the interaction of polyoxometallate solutions with various chemically modified surfaces. Specifically, we will use capital equipment purchased in year one of the grant to change the wavelength of the laser to the UV and investigate the interaction of phosphotungstic acid with the surfaces instead of the cobalt POM analog used to date. Work will also continue to definitively show that we are indeed making sulfonic acid coated fused silica.
