www.acsprf.org
Reports: DNI549041-DNI5: Molecular Modeling of Ionic Liquids Confined in Nanoporous Carbons
Francisco R. Hung, PhD , Louisiana State University
Abbreviations: electrochemical double-layer capacitor (EDLC), ionic liquid (IL), molecular dynamics (MD), mean squared displacement (MSD), multi-walled carbon nanotube (MWCNT)
During the past 12 months, my group has been focused on studying the structural and dynamical properties of several ILs confined inside nanoporous carbons using MD simulations. A fundamental understanding of the properties of ILs inside nanopores is relevant for applications of ILs as alternative electrolytes for energy storage in EDLCs. These devices have a structure similar to a battery, consisting of two carbon-based porous electrodes, an electrolyte and an ion-permeable separator. Because EDLCs exhibit fast charge/discharge times and can provide bursts of energy very quickly, they can complement the capabilities of internal combustion engines, fuel cells and batteries in hybrid vehicles, industrial equipment and electronic devices. Using ILs as alternative electrolytes allow EDLCs to operate at higher voltages, which results in improved energy storage and better charge/discharge times. Therefore, a fundamental understanding of the behavior of ILs inside nm-sized pores is crucial for the rational design of EDLCs. In particular, the structural properties of the confined ILs affect the capacitance in EDLCs, and the dynamical properties of ILs inside nanopores is one of the main factors determining the electrical resistance in an EDLC.
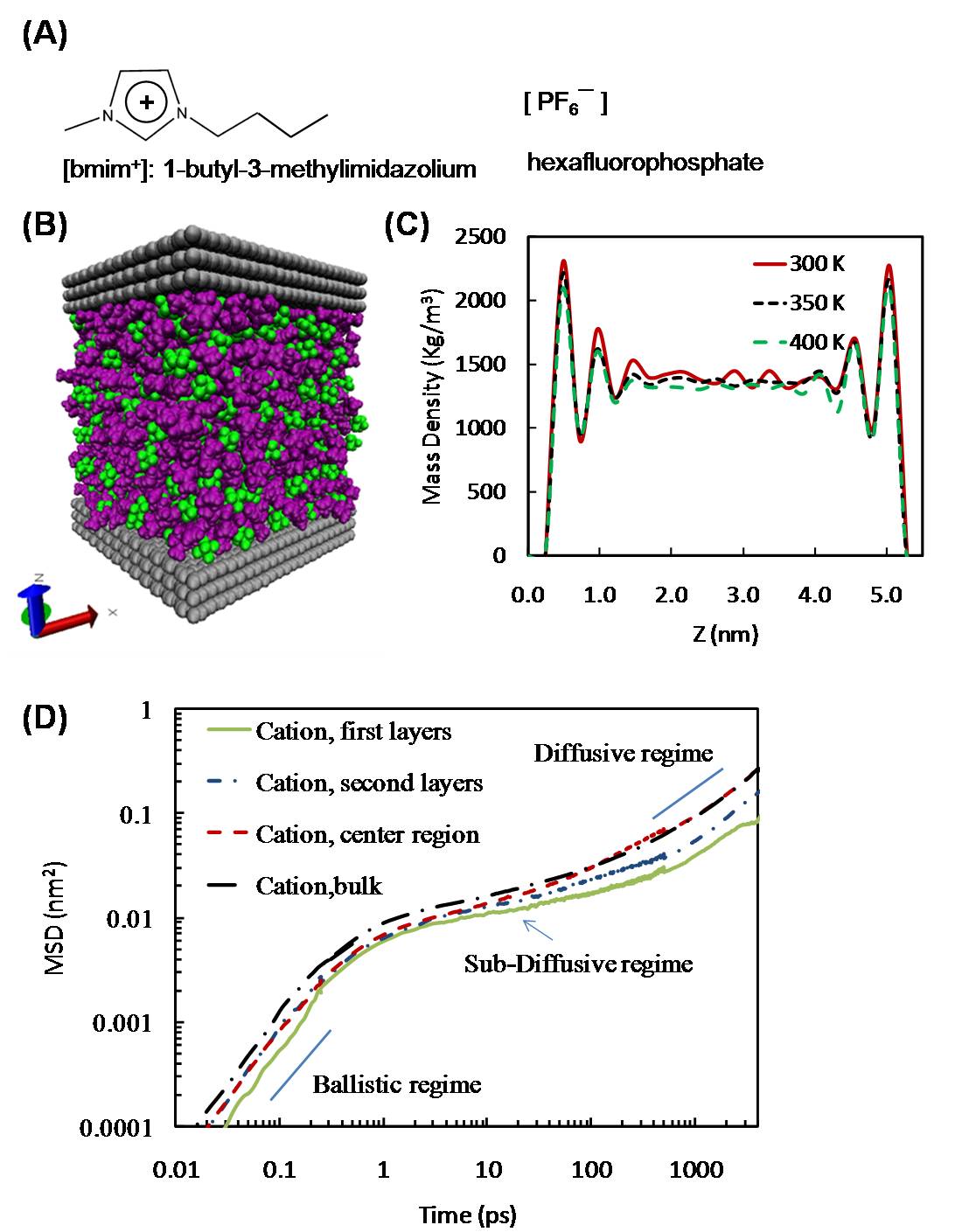
We completed an MD simulation study of the dynamical properties of the IL [bmim+][PF6-] (Fig. 1A) confined inside a slit-like graphitic nanopore of width 5.4 nm in the temperature range 300-400 K. Our results (Fig. 1B) indicate that the dynamics of the confined ions are highly heterogeneous and depend strongly on the distance of the ions with the pore walls. The ions in the center regions of the pore have dynamics and relaxation times that are very similar to those observed in a bulk IL at the same temperature. In contrast, the dynamics slow down appreciably as the ions get closer to the pore walls, and their relaxation times increase markedly. Our results also suggest that these dynamical heterogeneities are linked to differences in structural properties. Radial distribution functions suggest that the structure of the ions in the center of the pore is similar to that observed in the bulk IL; however, significant structural differences are observed between the ions near the pore walls and those in the center of the pore. It is interesting to compare these simulation results with those obtained by us in the past for the same IL confined inside a cylindrical MWCNT. Significant layering was observed for the IL inside the two different pore geometries. Likewise, in both pore geometries, in general the cations exhibited faster dynamics than anions, and the dynamics of the ions decrease monotonically as we go from the center of the pore to the IL layer near the pore walls. However, evidence of more complex dynamics was observed for the IL inside a cylindrical pore. MSD results observed for the IL inside a MWCNT of D = 3.0 nm (for which three layers of ions are observed) indicate that in the second layer, the MSD of the cations and anions are similar in magnitude; furthermore, the anions in this second layer have dynamics that are similar to those observed for the anions in the center of the pore. Evidence of similar complex dynamics was not observed for the same IL inside a slit pore. Therefore, the local dynamics of ILs confined inside nanopores are complex and heterogeneous.
Figure 1. (A) The IL [bmim+][PF6-]. (B) Snapshot from MD simulations of [bmim+][PF6-] in a slit-like graphitic nanopore, H = 5.4 nm. (C) Mass density profile of the IL along the z direction at different temperatures. We split the ions into three different layers/regions: the first layers (outer peaks in the figure, which are the closest to the pore walls), the second layers and the center regions. (D) Parallel component of the MSD of the cations at 300 K, as a function of their distance from the pore walls. The continuous blue lines depict the slopes expected for the ballistic and diffusive regimes. Similar results were obtained for the anions.
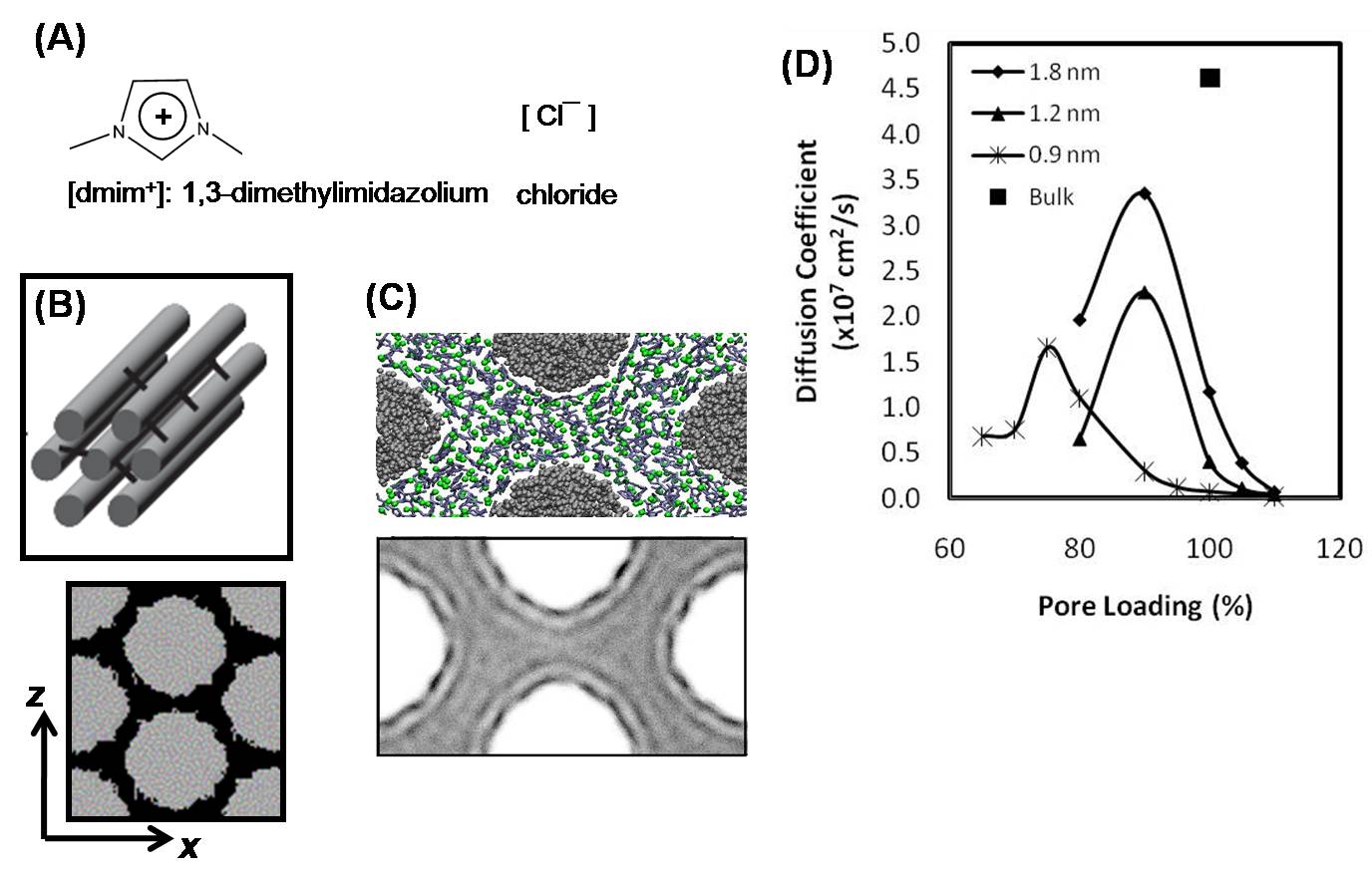
We have also conducted MD simulations of the IL [dmim+][Cl-] (Fig. 2A) confined inside nanoporous CMK-3 carbons. These are ordered mesoporous carbons consisting of hexagonally-packed nanorods with uniform diameters (Fig. 2B). These materials are made of amorphous carbon and exhibit walls with corrugations and curvature, and pores interconnected in a regular way. In CMK-3, the IL is adsorbed in the outer surfaces of the carbon nanorods (Fig. 2C). The density map of the ions shows strong layering behavior (Fig. 2D). For any given pore size, the axial diffusivity of the cations confined in CMK-3 reach maxima at pore loadings below ρbulk (Fig. 2E). In all cases, the dynamics of bulk IL are significantly faster than those of confined ILs. To the best of our knowledge, this is the very first simulation study of ILs confined in nanopores of geometry other than slit-like and cylindrical.
Figure 2. (A) Scheme of the IL [bmim+][PF6-]. (B) Schematic representation of the structure of CMK-3 (top), and front view of model CMK-3 (bottom, mean pore size ~ 1.4 nm). (C) MD simulations of [dmim+][Cl-] inside CMK-3 carbons: representative simulation snapshot (top), and density map of cations (bottom). The pore size of CMK-3 is equal to 1.8 nm, and the IL has a ρ ~ ρbulk. (D) Axial diffusivities of the cations at different pore sizes and pore loadings.
Monies from this grant have been used to partially support a postdoctoral researcher and a PhD student in my group. This student recently received a M.S. degree in Chemical Engineering while working towards obtaining his PhD degree. Support from this ACS-PRF grant has so far resulted in four publications, and one additional manuscript recently submitted for publication. Our work has been also featured in five invited talks at different universities (Penn State, Alabama, Mississippi State, Tulane and Université de Montpellier 2 in France), and in nine presentations at scientific conferences (e.g., AIChE 2009 and 2010 Annual Meetings, ACS Fall 2010 National Meeting).