www.acsprf.org
Reports: UNI150220-UNI1: Chiral Non-racemic Bicyclic Diketopiperazines: A Common Precursor to Explore Diverse Asymmetric Reactions
Jonathan R. Scheerer, Ph.D. , College of William and Mary
In the first year of funding from the Petroleum Research Fund, we devised a four-step synthesis of chiral non-racemic diketopiperazine 1 (60% overall yield) starting from serine methyl ester, an inexpensive commercially available reagent. This key bicyclic substrate has enabled studies into several stereoselective bond formations. In particular, we have focused our attention primarily on the diketopiperazine (DKP) Diels-Alder cycloaddition.
We have explored the fundamentals of the diketopiperazine (DKP) Diels-Alder cycloaddition using aza-diene 2, which can be prepared by DDQ oxidation of substrate 1 (eq 1). Study of the DKP Diels-Alder cycloaddition has been a rewarding and productive area of discovery. We have unveiled previously unknown (and in some cases surprising) reaction features. Notably, efficient reaction is observed in all substrates explored to date—both with electron rich and electron deficient alkene and alkyne substrates. Additionally we have determined that 1) the dominant product regioisomer is predictable, 2) stereochemistry favors reaction from the endo transition state with electron deficient a,b-unsaturated esters and imides, 3) excellent diastereofacial control is enforced with a removable aminal substituent. To this end, the cycloaddition of DKP aza-diene 2 provides a direct diastereoselective synthetic route to a variety of diazabicyclic structures. A manuscript summarizing these initial findings was recently published (Org. Lett. 2011, 13, 4430).
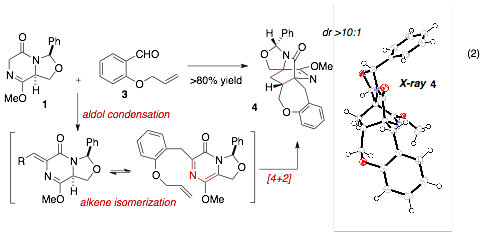
We recently developed a modified route to access reactive DKP aza-dienes via a multi-component reaction sequence involving an aldol condensation and alkene isomerization. When this reaction sequence is conducted in the presence of a suitable dieneophile, [4+2]-cycloaddition is observed. This cycloaddition can be templated from either an inter- or intramolecular manifold. For example, O-allyl salicaldehyde (3) can be combined with DKP 1 to give cycloadduct 4 in 80% yield (eq 2). The structure of 4 was verified by single crystal x-ray analysis. Similar to our preliminary studies (above), excellent diastereofacial selectivity is observed and cycloaddition occurs on the face opposite the aminal substituent. This sequence allows for rapid assembly of molecular complexity from simple starting materials. We are currently preparing a manuscript highlighting this domino reaction and its attributes, including the stereoselectivity, reactivity, and scope.