www.acsprf.org
Reports: DNI149707-DNI1: Low-Valent Iron-Catalyzed Transformations of Unsaturated Hydrocarbons
Tobias Ritter, PhD , Harvard University
Duringthe last ACS-PRF granting year, our laboratory reported progress toward developingwell-defined low-valent iron catalysts with our publication of the synthesis ofa well-defined iron precatalyst and its application in the 1,4-hydrosilylationof 1,3-dienes (J. Am. Chem. Soc. 2010, 132,13214–13216).
Previously, we reported two studiesusing iminopyridine-iron catalysts to functionalize 1,3-dienes: astereo- and regioselective 1,4-addition of terminal olefinsthat produced linear 1,4-dienes and a chemo-, regio-, and stereoselective 1,4-hydroborationto produce allylboranes. In both of these 1,3-diene functionalizationreactions, a ligand-dependent selectivity was observed and, in the latter case,was exploited to access both linear and branched allylboranes with highselectivity. The iminopyridine-iron catalysts provide access to versatilebuilding blocks that cannot be readily prepared by traditional syntheses or byother known transition metal-catalyzed reactions.
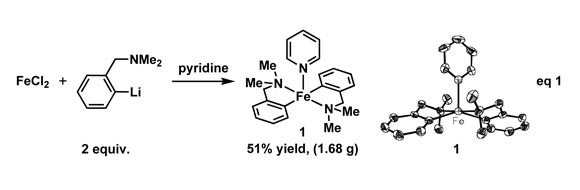
One of the major aims in ourresearch is to improve the understanding of carbonyl-free low-valent ironcatalysis. Towards this goal, we identified the need to develop well-definediron catalysts that would provide the opportunity for rational reactionimprovement. While low-valent transition metal catalysts have the advantage ofgeneral precatalysts such as Ni(COD)2 or Pd(dba)2, noanalogous complex exists for low-valent iron. Our previous reports usedactivated magnesium metal to reduce ferrous complexes in situ, generatingill-defined catalysts under homogeneous conditions not conducive to kineticanalysis. The strategy disclosed herein accesses low-valent Fe via reductiveelimination from a well-defined bis(aryl) iron(II) complex (1).
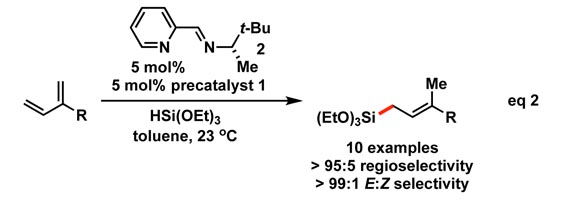
Complex 1 is readily synthesized from ferrouschloride, pyridine, N,N-dimethylbenzylamine, and n-butyl lithium (eq 1); its stability toward isolation andstorage at low temperature for months is likely due to the pseudo-trans arrangement of the carbon-based ligandsthat prevents spontaneous reductive elimination. An active iron catalyst,generated from 1upon addition of an exogenous ligand such as iminopyridine 2, was shown to effect1,4-hydrosilylation in high regio- and stereoselectivity (eq 2). Access to precatalyst1 allowed forthe ready evaluation of different ligands to identify iminopyridines thatproduced linear allylsilanes in high selectivity. Our hydrosilylation reactionprovides access to linear allylsilanes in higher chemo-, regio- and stereoselectivitythan known allylmetal addition reactions and other transition metal-catalyzedmethods.
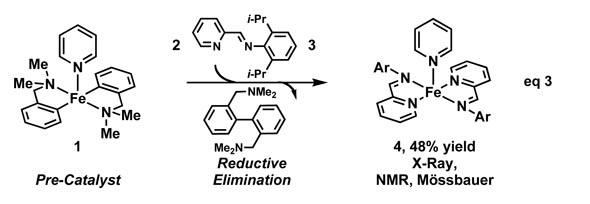
Upon addition of iminopyridine 3, a neutral iminopyridine complex, 4, was isolated and characterized byX-ray crystallography, NMR, and Moessbauer spectroscopy (eq 3). Previousstudies by Wieghardt and coworkers have shown that iminopyridine ligands areredox active and can accept electrons from electron-rich metals; iminopyridineligands were selected because they could potentially lower activation barriersby acting as electron reservoirs during catalysis. Spectroscopic evidence formetal–ligand redox participation in complex 4 suggests that it is more accuratelyrepresented as an Fe(II) center coordinated to two radical anion ligands.Complex 4 itselfis a precatalyst for the 1,4-hydrosilylation reaction; reaction with 4 is homogeneous, well-behaved, andcan be followed kinetically. Analysis of the kinetic profiles of the reactionwith precatalyst 4in the presence and absence of exogenous ligands showed that addition ofpyridine and iminopyridine 3 retarded the rate of reaction. In comparison, addition ofonly 1 equiv of iminopyridine 2 to precatalyst 1 produced a catalyst that was more active than when 2 equivof iminopyridine 2were added. Thus, an advantage of using precatalyst 1 is that it can be used in situwithout the need to isolate and purify the iminopyridine complex, as with 4.
In summary, support from the ACS-PRFhas allowed us to make progress toward the two major aims outlined in ourproposal: (1) to develop iron-catalyzed transformations toaccess building blocks from commodity chemicals such as dienes, which can bedirectly derived from petroleum and (2) to improve understanding ofcarbonyl-free low-valent iron catalysis. The first aim was fulfilled in ourdevelopment of a regio- and stereoselective 1,4-hydrosilylation of 1,3-dienes;we have made progress towards the second aim by isolating and characterizing animinopyridine-Fe complex that allows kinetic analysis of the hydrosilylationreaction.