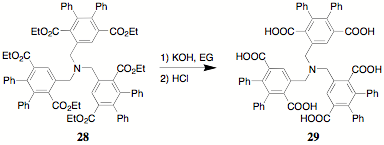AmericanChemicalSociety.com
Reports: B1 46413-B1: The Synthesis of Novel Indenofluorenes
William A. Feld, Wright State University
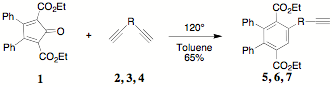
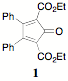
Carboxylated cyclopentadienones (CCPD) react readily in Diels-Alder reactions exhibiting inverse electron demand (dienophile is electron rich). This presents many intriguing possibilities and provides access to a wide variety of new richly functional organic systems. The reactions outlined in previous reports set the stage for the current report of 1) the use of 2, 5-dicarboethoxy-3,4-diphenylcyclopentadienone 1 ("Orange") as a convenient precursor to pendent ethynyl containing systems, 2) the use of 2, 5-dicarboethoxy-3,4-bis(2-fluorophenyl)cyclopentadienone 1 to probe steric effects and 3) the use of "Orange" in the preparation of hydroxy/alkoxy substituted terephthalates.
The
original report of a fortuitous calculation error in the use of propargyl ether
2 as a Diels-Alder reactant with 1 prompted a further look at the use of related
diynes 1,5-hexadiyne 3 and
1,5-octadiyne 4 in this
reaction. The study found that the original 1:10 ratio of 1:2
could conveniently be reduced to 1:3 without compromising the isolation of the
mono reaction products, the terephthalates 5 (R = -CH2OCH2-, 6 (R = -(CH2)2-) and 7 (R = -(CH2)4-). The use of 5, 6
and 7 in the preparation of
ethynyl substituted poly(phenylene vinylene)s by subsequent preparation of the
corresponding bis(chloromethyl) derivatives is anticipated. The incorporation
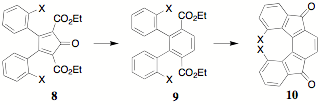
of substituents in the benzil portion of CCPDs, particularly at the 2 position,
as shown in 8, was a focus as they are
precursors to terephthalates 9
which can undergo ring closure to indenofluorendiones 10. It was anticipated that steric factors in 9 and 10
could conveniently be used as a probe of the efficiency of the ring closure
reaction in spite of the potential for the substituents to, in fact, prevent
the ring closure. We earlier reported
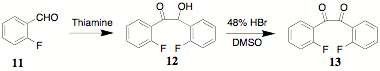
(2009 PRF) that the synthesis of bis(o-fluoro)benzil 12 could be accomplished by the use of thiamine in
place of cyanide ion in the benzoin condensation of 2-fluorobenzaldehyde 11. Thus, 2,2'-difluorobenzoin 12 was synthesized in excellent yield from
2-fluorobenzaldehyde 11. Benzoin
12 was originally oxidized by
the use of ammonium nitrate and copper (CAUTION: nitrogen evolution was sudden
and spontaneous). An alternative oxidation (DMSO, conc HBr) provides 13 in excellent yield with considerably less
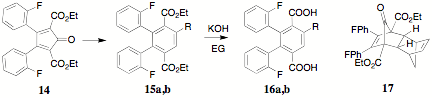
uncertainty and/or danger. Subsequently, 13 was converted to the cyclopentadienone 14. The terephthalates 15a,b (a,
R = -H; b, -C6H13) were
prepared by a Diels-Alder reaction of 1 with norbornadiene or 1-octyne, respectively. Interestingly, diester 15a could be alternately generated in quantitative
yield by a two-step process involving the bicyclic adduct 17 and its melt pyrolysis. The diesters 15a,b were hydrolyzed with potassium hydroxide in
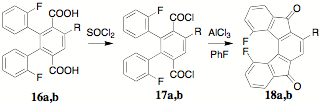
ethylene glycol to provide the corresponding diacids 16a,b. Ring closure of the diacids 16a,b to the corresponding indenofluorendiones 18a,b was unsuccessful using conc. H2SO4.
Unlike the unsubstituted version, decomposition of the starting material
ensued. However, the conversion of the
diacids 15a,b to the corresponding
diacid halides 16a,b provided a
pathway to the indenofluorendiones 18a,b. The 1H
NMR spectra of 15a,b – 18a,b clearly indicate the presence of stereoisomers as
well as extensive fluorine coupling. Because of restricted rotation because of
the presence of the fluoro substituents, both the cis and trans isomers can be clearly seen but not conclusively
identified. By analogy to similar systems, the trans isomer appears to be the
dominant isomer. Crystallographic data indicates a mixture of the diastereomers
for 15a,b and is being further investigated. More study is also
needed to ascertain the structural information provided by the 1H, 13C
and 19F NMR spectra for 15a,b
– 18a,b. The obvious
through space interaction of the fluorines in 18a,b and the potential left- and right-handed helical
possibilities for these systems is of considerable interest. As
part of our goal to generate polyfunctional indenofluorendiones, alternate core
connection schemes were investigated and we reported (2009, PRF) that the
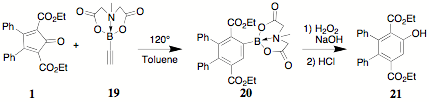
phenolic terephthalate 21 could be
synthesized using the MIDA ester 19
(commercially available from Sigma-Aldrich). The cost of 19 (~$190/g), the obvious lack of "atom economy" and
the complicated synthesis of 19
prompted us to reinvestigate the synthesis of 21 by a procedure
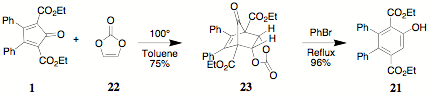
involving vinylene carbonate 22 (as
reported in the literature). Vinylene carbonate, while not "cheap" does provide
a measure of "atom economy." The reaction of 1 and 21
generates the bicyclic intermediate 23. Thermolysis of 23 in
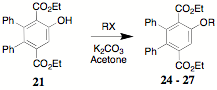
bromobenzene provides a convenient, reliable preparation of 21 in contrast to the reported yield. The alkylation
of 21 employing a phase transfer
protocol results in the generation of the ethers 24 (R = Me), 25 (R = Allyl), 26 (R = Propargyl) and 27 (R = Benzyl). The construction
of teathered poly(terephthalates) like 28
was a major objective of this project and prior reports delineated its
synthesis. In keeping with our ultimate goal of generating
poly(indenofluorendiones), the hydrolysis of 28 was carried out in ethylene glycol with potassium
hydroxide to yield the hexaacid 29.
The characterization of 29 has
proven to be a formidable task. The
reactions outlined in this report and those of previous reports, add to the
knowledgebase of reactivity and reaction diversity for CCPDs, namely 2,
5-dicarboethoxy-3,4-diphenylcyclopentadienone 1 ("Orange") and the bis(o-fluoro)
derivative 14. A wide
variety of functionalized terephthalates, teathered terephthaltes and highly
sterically hindered indenofluorendiones can be conveniently generated in
excellent isolated yields.
Copyright © American Chemical Society