AmericanChemicalSociety.com
Reports: B6 46291-B6: Condensed Phase Effects on the Structural Properties of Friedel-Crafts Intermediates: RF-BF3
James A. Phillips, University of Wisconsin (Eau Claire)
Context: The main objective of this project is to characterize the structural properties of organofluoride – boron trifluoride complexes (RF'–BF3) in the gas-phase and in bulk, condensed-phase environments via computations and low-temperature infrared spectroscopy. These species are key intermediates in Friedel-Crafts reactions [1] - an important class of carbon-carbon forming processes that facilitate the conversion of petroleum feedstocks to commercially-viable compounds. One common example is the alkylation of benzene, viz.
It has been presumed for 50 years
that the first step is the formation of a 1:1 RF–BF3
intermediate complex [1], but it is also known that the methyl complex is quite
weak in the gas phase with an experimental B-F' distance of 2.42 [2]. Thus,
its solution-phase reactivity of these 1:1 complexes can only be rationalized
by invoking a substantial structural re-arrangement that results from
interactions with the solvent.
Gas-Phase Structures and Binding Energies
(CH3)3C-BF3
B-F'
potentials: Gas phase and dielectric media
(CH3)3CF–BF3
Experimental
and computed IR spectra for (CH3)2CH–BF3
Molecular Cooperativity:
the Influence of a Second RF Subunit 1. Olah, G.A.
Friedel-Crafts and Related Reactions; Wiley/Interscience: New York, 1963. 2. Leopold, K.R.; Canagaratna, M.; Phillips, J.A. Accts. Chem. Res. 1997, 30, 57. 3. Reed, A.E.; Curtiss,
L.A.; Weinhold, F. Chem. Rev. 1988, 88, 899. 4. Bader, R.F.W. Atoms in Molecules - A Quantum Theory;
Oxford University Press: Oxford, 1990. 5. Miertus, S.; Tomasi, J. Chem. Phys, 1982, 65, 239. 6. van der
Veken, B.J.; Sluyts, E.J. J. Phys. Chem. A. 1997, 107, 9070.
![]()
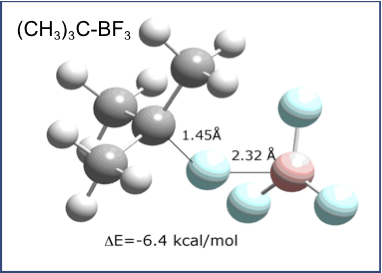 Previously,
we reported the calculated (X3LYP/aug-cc-pVTZ) gas-phase structures of CH3F–BF3,
(CH3)2CHF–BF3, (CH3)3CF–BF3,
which exhibited long B-F' distances (2.35 - 2.41), and a MP2/aug-cc-pVTZ
binding energies of 3.6 to 6.4 kcal/mol. The structure of the t-butyl complex
is shown at the left.
Previously,
we reported the calculated (X3LYP/aug-cc-pVTZ) gas-phase structures of CH3F–BF3,
(CH3)2CHF–BF3, (CH3)3CF–BF3,
which exhibited long B-F' distances (2.35 - 2.41), and a MP2/aug-cc-pVTZ
binding energies of 3.6 to 6.4 kcal/mol. The structure of the t-butyl complex
is shown at the left. 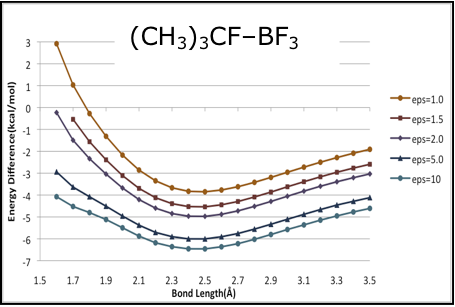 Calculated
gas-phase (X3LYP/aug-cc-pVTZ) B-F' potential curves for CH3F–BF3,
(CH3)2CHF–BF3
and (CH3)3CF–BF3, are shown in the
figures below. The gas-phase potentials (top traces in each curve) show no
major peculiarities, but are rather soft long the inner wall, such that the
energy only rises by about 2.5 kcal/mol over several
tenths of an between the minima and the inner wall. The curves computed for
dielectric media (via PCM [5]), do differ notably
between the methyl complex and the two larger systems. As the dielectric
increases, the potential of CH3CF–BF3 responds
uniformly across all bond lengths, such that there is no change in shape, and
no signs that a distinct structure with a shortened B-F' bond length is
stabilized by the medium. For the larger complex however, the inner portion of the
curve is preferentially stabilized. For (CH3)2HCF–BF3
and the potential at 1.7 is only about 1 kcal/mol above the minimum.
Calculated
gas-phase (X3LYP/aug-cc-pVTZ) B-F' potential curves for CH3F–BF3,
(CH3)2CHF–BF3
and (CH3)3CF–BF3, are shown in the
figures below. The gas-phase potentials (top traces in each curve) show no
major peculiarities, but are rather soft long the inner wall, such that the
energy only rises by about 2.5 kcal/mol over several
tenths of an between the minima and the inner wall. The curves computed for
dielectric media (via PCM [5]), do differ notably
between the methyl complex and the two larger systems. As the dielectric
increases, the potential of CH3CF–BF3 responds
uniformly across all bond lengths, such that there is no change in shape, and
no signs that a distinct structure with a shortened B-F' bond length is
stabilized by the medium. For the larger complex however, the inner portion of the
curve is preferentially stabilized. For (CH3)2HCF–BF3
and the potential at 1.7 is only about 1 kcal/mol above the minimum.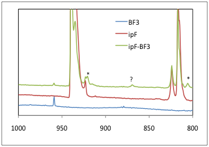 The
figure at the left shows the C-F stretching region of the IR
spectrum of (CH3)2CHF–BF3
in solid Ne, for which the peaks assigned to the 1:1 complex are marked with
asterisks. The top trace is the sample containing both isopropyl fluoride and
BF3, the bottom two traces are controls that contain only BF3
or (CH3)2CHF. These peaks, both
assigned to modes that involve some degree of C-F stretching motion, agree with
our DFT predictions (X3LYP/aug-cc-pvTZ) for the
gas-phase complex to within 3 cm-1. This
provides a great deal of validation for our characterization of the gas-phase complex,
and the at best meager effects of low-dielectric media.
The
figure at the left shows the C-F stretching region of the IR
spectrum of (CH3)2CHF–BF3
in solid Ne, for which the peaks assigned to the 1:1 complex are marked with
asterisks. The top trace is the sample containing both isopropyl fluoride and
BF3, the bottom two traces are controls that contain only BF3
or (CH3)2CHF. These peaks, both
assigned to modes that involve some degree of C-F stretching motion, agree with
our DFT predictions (X3LYP/aug-cc-pvTZ) for the
gas-phase complex to within 3 cm-1. This
provides a great deal of validation for our characterization of the gas-phase complex,
and the at best meager effects of low-dielectric media.
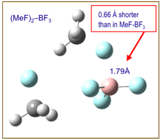 At
this point in the project, we are faced with a striking discrepancy.
Conductivity measurements indicate that some sort of ionization occurs when BF3
is added directly to alkylfluorides (with R >
ethyl), yet our theoretical characterization of the 1:1 complexes suggests that
spontaneous ionization should not take place – even in fairly polar
solvents. Moreover, our experimental frequencies indicate that our theoretical characterizations
of the 1:1 complexes are reasonable, at least for the gas-phase and low
dielectric environments. This paradox led us to consider the role of a second
alkyl fluoride molecule, which may act mediate atom transfer to form ions. The
graphic at the left displays an initial result from our attempt to characterize
a series of 2:1, i.e. (RF2)–BF3 complexes via
density functional calculations. This shows that the addition of a second CH3F
subunit causes the B-F' distance to contract by about 0.66. This is a
cooperative, molecular effect that is not predicted using continuum solvation
models.
At
this point in the project, we are faced with a striking discrepancy.
Conductivity measurements indicate that some sort of ionization occurs when BF3
is added directly to alkylfluorides (with R >
ethyl), yet our theoretical characterization of the 1:1 complexes suggests that
spontaneous ionization should not take place – even in fairly polar
solvents. Moreover, our experimental frequencies indicate that our theoretical characterizations
of the 1:1 complexes are reasonable, at least for the gas-phase and low
dielectric environments. This paradox led us to consider the role of a second
alkyl fluoride molecule, which may act mediate atom transfer to form ions. The
graphic at the left displays an initial result from our attempt to characterize
a series of 2:1, i.e. (RF2)–BF3 complexes via
density functional calculations. This shows that the addition of a second CH3F
subunit causes the B-F' distance to contract by about 0.66. This is a
cooperative, molecular effect that is not predicted using continuum solvation
models.
Copyright © American Chemical Society

