AmericanChemicalSociety.com
Reports: GB1 47674-GB1: A New Solution Phase Protecting Group Strategy for Alkyl Guanidines
Janet A. Asper, University of Mary Washington
Progress
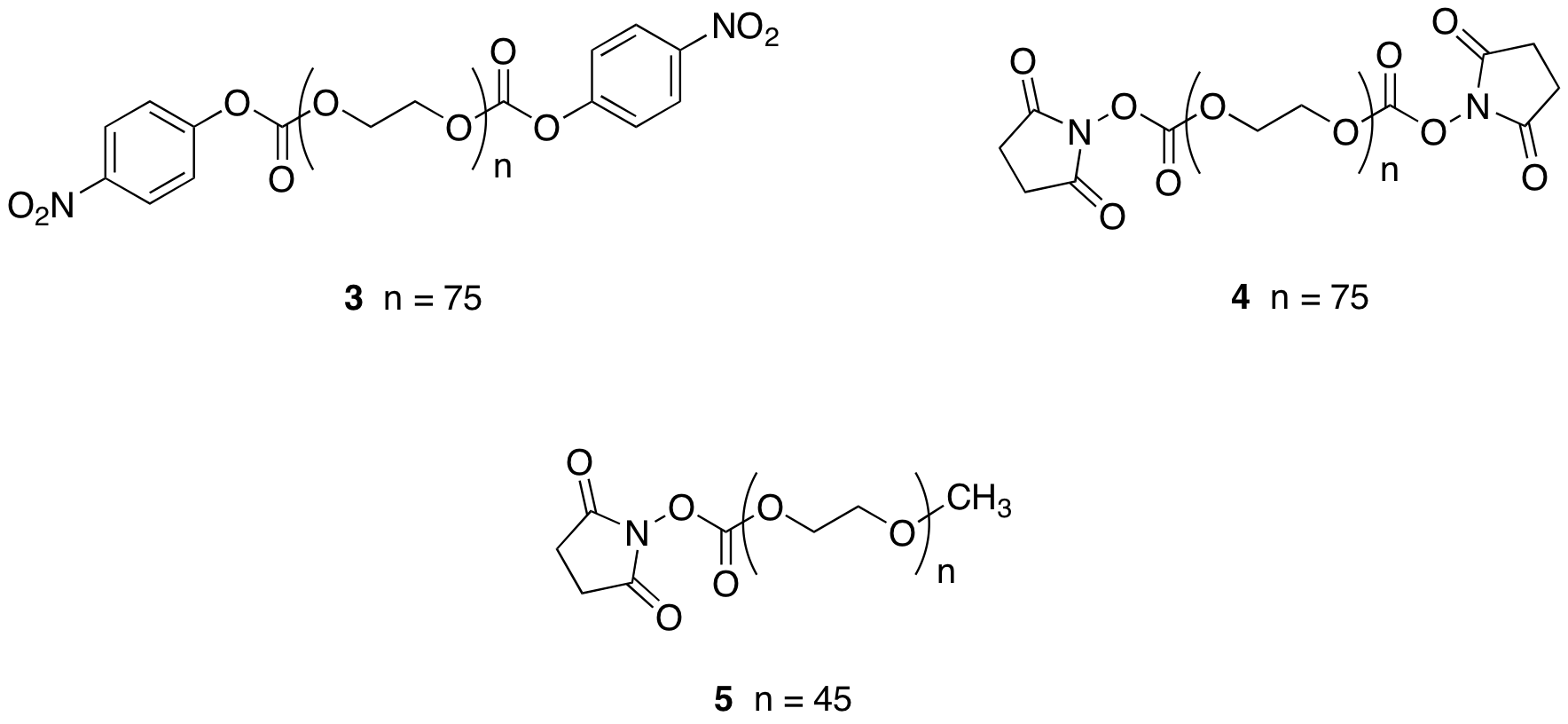
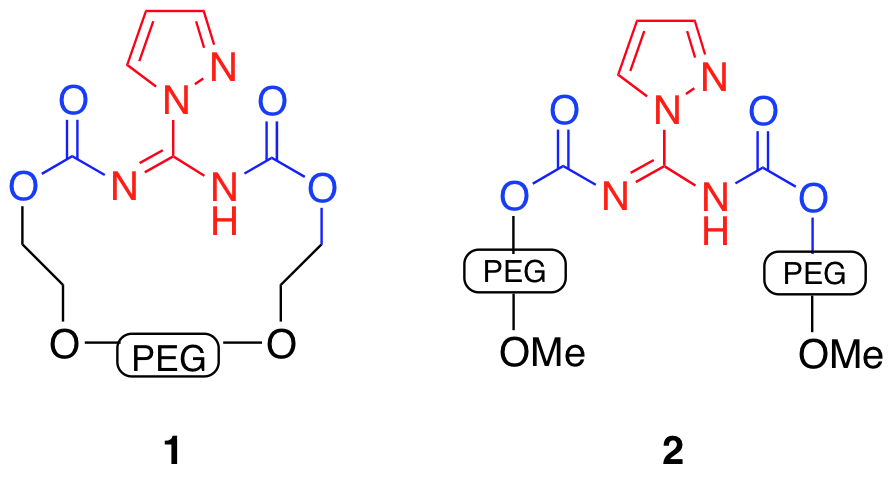
During the 2009-2010 grant year, we continued to develop the poly(ethylene glycol) (PEG) tethered guanidnylation reagents (Figure 1). We have made progress in exploring the preparation and usability of activated poly(ethylene glycol) polymers and began coupling those PEGs to our guanidinylation reagents. Our institution now has a high field NMR, which will greatly facilitate the characterization of these molecules.
Figure 1. Proposed
guanidinlation reagents.
We are using two commercially available
PEGs for our research: a
difunctional PEG with an average weight of 3350 (PEG 3350) and a monofunctional
PEG monomethyl ether with average molecular weight of 2000 (PEG-2000). PEG-3500-dinitrophenyl carbonate, 3, has been prepared in 60.3% yield
using traditional methods, and in 20.3% unoptimized yield under solvent free,
microwave conditions. These
preliminary microwave results are encouraging, as they support our hypothesis
that our PEG based protective group may not only allow for the use of liquid
phase organic synthesis techniques, but also allow for microwave based solvent
free techniques.
Figure 2. Activated
poly(ethylene glycol) carbonates.
Succimidiyl carbonate activated
PEGs were also prepared. In these reactions, there were difficulties in the
solubilities of the reagents, and several different literature preparations
were attempted.1,2 At
this point, we have synthesized the difunctional succimidyl carbonate PEG-3350,
4, and monofunctional succimidyl
carbonate PEG-2000, 5, in 32.1% and
22% unoptimized yields, respectively.
During preparation of these compounds, we learned a great deal about the
nature of PEG based reagents. For
liquid phase organic synthesis reactions to work well, the polymer-based
reagent must be soluble enough in the reaction solvent for the desired
reactions to take place, but insoluble enough in a different solvent for
product isolation upon precipitation.3 It appears that most of the
product loss was in purification steps, and we are currently working to improve
those yields
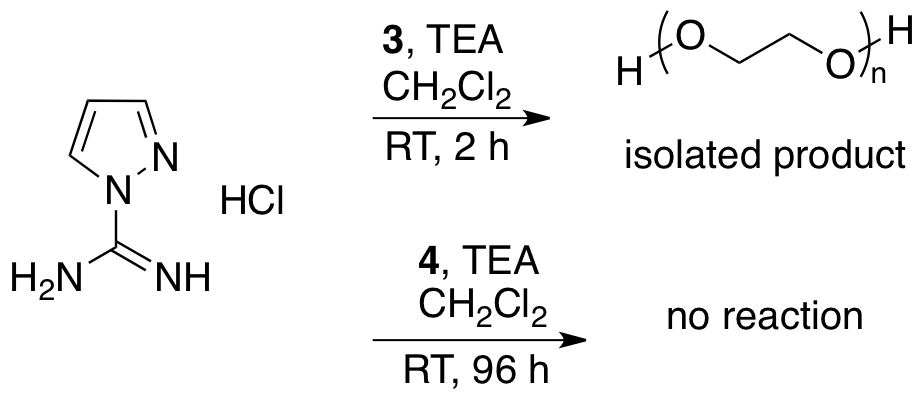
Undergraduate Researcher Katie
Strong focused on preparation of the guanylpyrazole based guanidinylation
reagent, 5, as its UV activity makes
the reactions much easier to follow using TLC. The reaction of guanylpyrazole hydrochloride with the
nitrophenyl carbonate activated PEG resulted in cleavage of the activating
group. Reaction with the succimidyl
carbonate activated PEG, afforded unreacted activated PEG as the only polymeric
product. These reactions will
continue to be studied next year. Characterization of these large compounds
with our 60 MHz NMR was very difficult, but with our new 300 MHz NMR, we
anticipate making great progress during our grant extension.
Figure 3. Synthesis of
guanidinylation reagents.
Impact The impact of the PRF grant on my
career and on my students has been so great that it is almost impossible to
measure. Although UMW is a
teaching institution, it is a requirement for tenure that faculty have demonstrated
a pattern of scholarly activity that contributes to our discipline beyond the
campus. Two of the activities that
are considered "significant" in building that pattern are winning a grant and
participation in programming at a professional meeting. Winning this grant, and
using the funds to travel to and present at the ACS National Meeting in San
Francisco were recognized as significant contributions to my pattern of
professional activity. I have been
recommended for tenure by the Promotion and Tenure Committee at UMW, and am
awaiting recommendations from the Dean, Provost, President and Board of
Visitors.
In 2009, UMW entered into an
educational partnership agreement with chemists at the Naval Surface Warfare
Center, Dahlgren, VA. The main
component of this agreement is that UMW houses and maintains a 300 MHz NMR
owned by the Navy. I believe that
my success in obtaining the PRF Grant helped me to convince the UMW
administration that partnering with the Navy was a good idea, and prove that I am
capable of obtaining and maintaining the agreement and the instrument. The NMR was installed in July of 2010,
and has accelerated progress on this project this year.
During the 2009 - 2010 grant
year, I used PRF funds to take my undergraduate researcher, Katie Strong, to
the ACS National Meeting in San Francisco and present at her work in the
undergraduate poster session. Katie was very excited by the scope of the
meeting, all of the chemistry that she saw, and the students that she met during
the undergraduate activities. In
addition, Katie's work on this project was the core of her Honors Thesis, which
she successfully defended in April of 2010. She applied to several graduate
schools, and was accepted to her first choice, Emory University. Katie keeps in touch, and is always
telling me how the experience that she gained presenting at the national
meeting and preparing a honors thesis make her feel "ahead of the curve" in her
research and presentation experiences in her first year of graduate
school.
1 Miron, T.; Wichek, M. Bioconj.
Chem 1993, 4, 568-569.
2 Sarvi, F.; Vasheghani-Farahani, E.;
Shojaosadti, S.A.; Hashemi-Najafabadi, S.; Moin, M.; Pourpak, Z. Iranian
Polymer Journal 2006, 4, 525.534.
3
Gravert, D. J.; Janda, K. D. Chem. Rev. 1997, 97, 489-510.
Copyright © American Chemical Society