AmericanChemicalSociety.com
Reports: UR1 50115-UR1: Expanding the Scope of the Diels-Alder Reaction: Development of Cationic Dienophiles Stabilized by Cobalt-Complexed Alkynes
Kevin M. Shea, Smith College
As described in my original proposal, the goal of this project is to develop a new class of cationic Diels-Alder dienophiles. Our guiding principle is to exploit the cationic stabilizing ability of cobalt-complexed alkynes, and our hypothesis is that cationic dienophiles incorporating cobalt will react faster and more efficiently with a variety of dienes. We planned to examine the reactivity of several cationic dienophiles and then explore their reactivity in tandem Diels-Alder/Pauson-Khand reactions for the synthesis of complex polycycles.
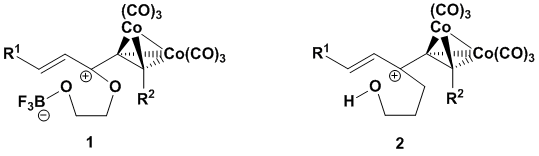
Our initial investigations focused on the development of two different classes of dienophiles: Gassman (1) and non-Gassman (2) types. Named for Paul Gassman, the pioneer in the study of cationic dienophiles, our Gassman-type dienophiles incorporate an oxygen atom that, along with the cobalt-complexed alkyne, stabilizes the reactive cation. In contrast, the non-Gassman-type dienophiles have a cation only stabilized by an adjacent cobalt-complexed alkyne. We have developments to report on both of these fronts during the first year of our PRF-funded project.
Scheme 1. Examples of a Gassman- and a non-Gassman-type dienophile
We spent a significant amount of
time working to establish a reliable synthesis of a non-Gassman-type
dienophile. Unfortunately, our initial
synthetic route proved completely unreproducible, and
we were forced to abandon it. The route
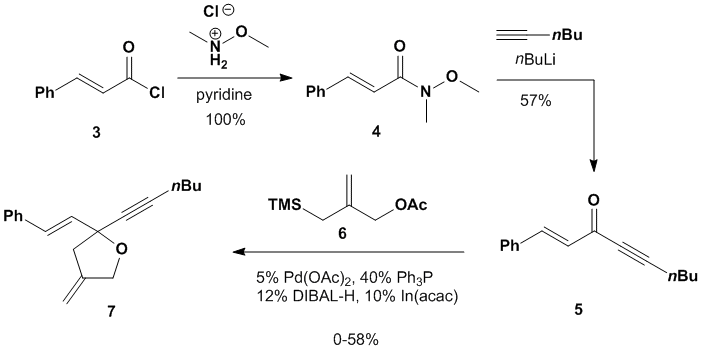
is outline in Scheme 2 and involves repeating a synthesis published by Trost in
1993.[1] The first two steps were straightforward;
synthesis of Weinreb amide 4 and
conversion to enyne 5 proceeded uneventfully.
The results of the next step, the key step in the route, were highly
variable in our hands and rarely produced greater than 20% yield of the target,
tetrahydrofuran derivative 7. We attempted this reaction many times and
verified the purity of all of the required reagents. We once obtained a 58% yield but were never
able to reproduce it. It is also
challenging to purify the desired target from the reaction mixture via column
chromatography. So, in light of all of
these obstacles, we plan to turn our attention to a non-Gassman-type dienophile
that is much easier to prepare.
Scheme
2. Synthetic route to a non-Gassman-type dienophile Our previous target, compound 7, is the only known molecule
containing our desired arrangement of a tetrahydrofuran substituted in the
2-position with both an alkene and an alkyne.
We imagined several other routes to tetrahydrofuran derivatives, but all
seemed lengthy and not completely straightforward. Our apparent fascination with cyclic ether
substrates stemmed from Gassman's results where he demonstrated superior yields
in Diels-Alder reactions with oxonium ion dienophiles derived from cyclic
acetals versus the corresponding acyclic acetals.[2] Considering our difficulties obtaining the
synthetically challenging cyclic ether targets, we plan to turn our future
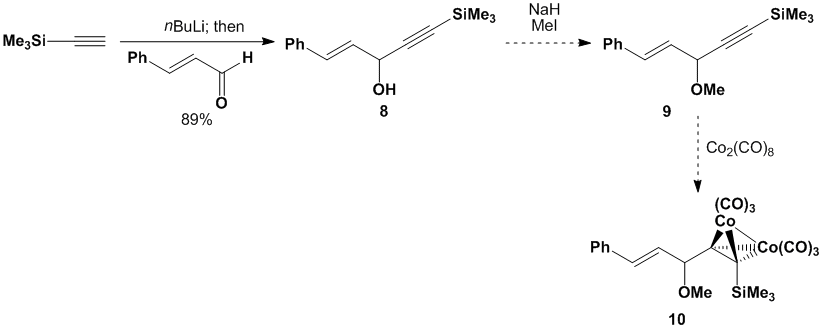
studies on non-Gassman-type dienophiles to acyclic ethers (like 10).
We already prepare the one corresponding alcohol (8) for our Gassman type dienophile studies and conversion to ether
substrate 9 should be facile. We are anxious to synthesize dienophile
precursor 10 and to evaluate its
behavior as a Diels-Alder dienophile versus the non-complexed ether 9.
Scheme
3. Proposed synthesis of acyclic non-Gassman-type
dienophile Our investigations into Gassman-type
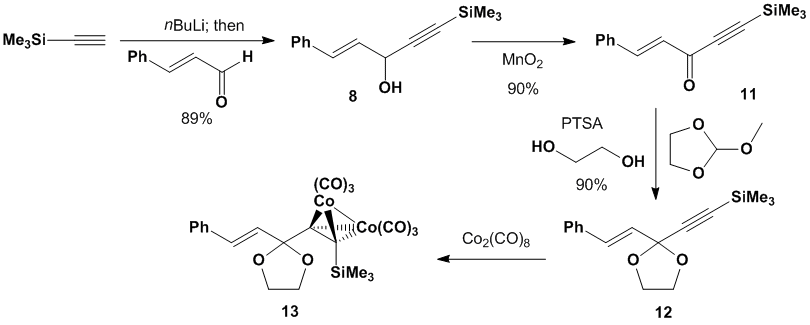
dienophiles is progressing more smoothly since synthesis of the key substrate
is much less challenging. We have a
robust synthesis of cobalt-containing dienophile as outlined in Scheme 4. Acetylide addition
to cinnamaldehyde furnishes alcohol 8 (in
89% yield) which is subsequently oxidized in good yield to provide ketone 11.
Ketalization with ethylene glycol yields ketal 12, and
subsequent treatment with dicobalt octacarbonyl
provides dienophile precursor 13. Scheme
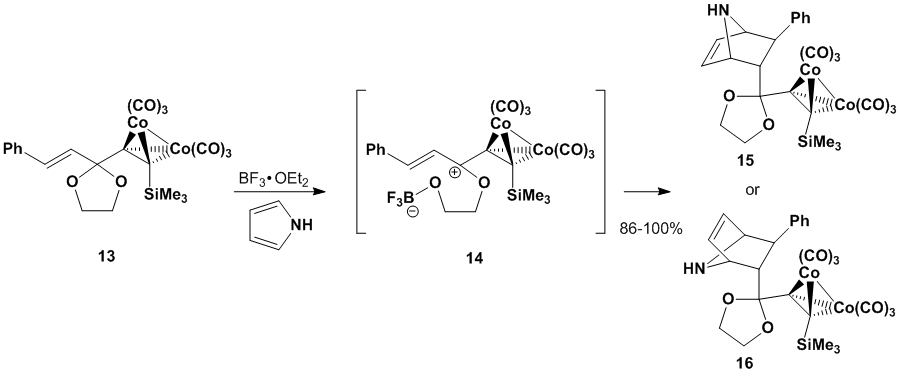
4. Synthesis of Gassman-type dienophile Combination of dienophile precursor
13 with BF3•OEt2
and several dienes yields Diels-Alder products.
As highlighted in Scheme 5, the resulting cationic dienophile 14 combines efficiently with pyrrole to
yield one product diastereomer, either endo product 15 or exo product 16. (Our NMR spectra for the product show no
evidence for the production of multiple diastereomers.) Uncomplexed dienophiles lead only to
decomposition of the dienophile under the reaction conditions.
Scheme
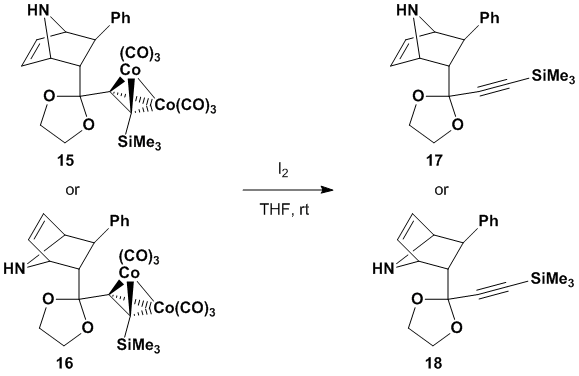
5. Diels-Alder reaction of a Gassman-type dienophile To date, we have been unable to
determine if the reaction generates isomer 15
or 16. We were hampered in our structure
determination efforts by two important factors.
First, organometallic cobalt complexes are known to be difficult to
analyze using NMR. Sometimes, they lead
to perfect spectra; other times, paramagnetic impurities generated by slow
decomposition of the cobalt-complexed alkyne make it impossible to lock on a
sample. Unfortunately, for compound 15/16,
we could not obtain high-resolution spectra that would enable unambiguous
structure identification. Second, the
first year of our award corresponded with major changes in the Smith chemistry
department's NMR holdings. During the
summer of 2010, when the bulk of this research was conducted, we were in the
middle of switching from a 15 year old 400 MHz JEOL instrument to two new 300
MHz and 500 MHz Bruker spectrometers. The latter problem will be easily remedied
during the second year of our investigation.
Formal training with Bruker scientists is
already scheduled and will enable us to deploy the full power of these instruments
to answer our structural question. The
former issue, paramagnetic impurities inherent in the samples, will not be
solved by using our new NMRs. Instead,
we investigated a variety of cobalt-decomplexation techniques to determine the
best method for our system. We quickly
settled on an iodine oxidation method for the production of cobalt-free
material (see Scheme 6). We are
confident with this material in hand and our new spectrometers that we will be
able to unambiguously determine the product structure.
Scheme
6. Cobalt decomplexation of Diels-Alder products During the next year of our
investigation, we will focus on reactions with other dienes that will enable continued
investigations into the regio- and stereoselectivity
of our novel dienophile.
[1] Trost, B. M.; Sharma, S.; Schmidt, T. “In(+3) as a Chemoselectivity Switch for TMM-PD L2 Cycloadditions to Ynones,” Tetrahedron Lett. 1993, 34, 7183-7186.
[2] Gassman, P. G.; Singleton, D. A.; Wilwerding, J. J.; Chavan, S. P. “Acrolein Acetals as Ally Cation Precursors in the Ionic Diels-Alder Reaction,” J. Am. Chem. Soc. 1987, 109, 2182-2184.
Copyright © American Chemical Society