AmericanChemicalSociety.com
Reports: B1 48367-B1: Beta-Hydroxysalicylhydrazones: Chiral, Non-Racemic Tridentate Catalysts for Asymmetric Synthesis
Shawn R. Hitchcock, Illinois State University
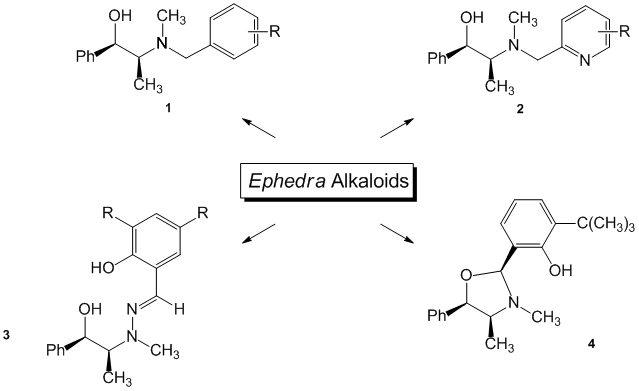
In the previous granting period, our interest was focused on the development of Ephedra alkaloids as ligands for the catalytic asymmetric addition of diethylzinc to aldehydes and diphenylphosphinoylimines (Figure 1). The diethylzinc addition reactions were of interest for our group, provided technical training for graduate students, undergraduate students, and an ACS project SEED student.
Figure 1. Ephedra alkaloids for use in the asymmetric addition of dialkylzincs to aldehydes and imines.
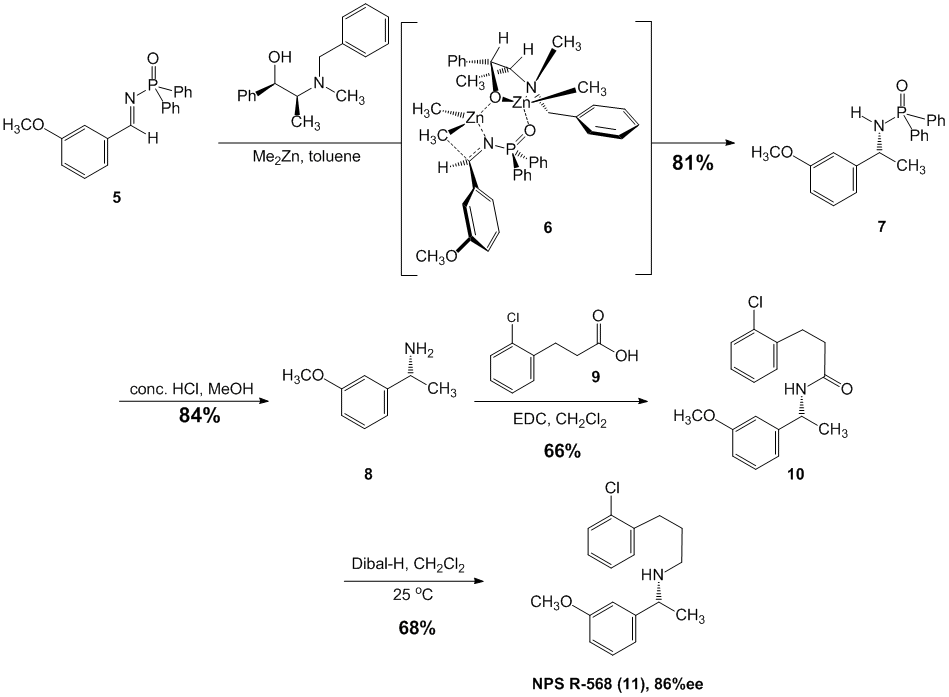
In fact, our last major publication involving diethylzinc involved the synthesis of the calcimimetic agent NPS R586. This was the first dialkylzinc-Ephedra ligand promoted organometallic transformation used for this process. The enantiomeric ratio of the final target was determined to be 93:7 (86% ee) favoring the (R)-enantiomer by derivatization and chiral stationary phase HPLC analysis (Scheme 1).
Scheme 1. Synthesis of the calcimimetic agent NPS R-568.
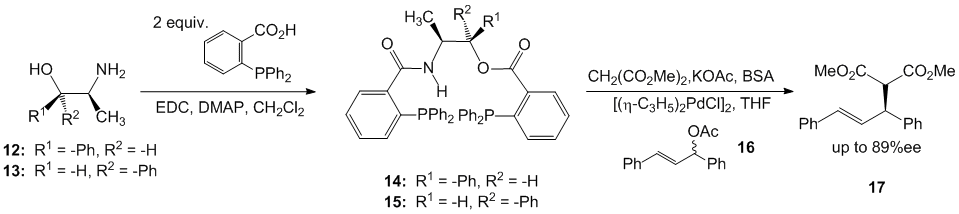
There was an evolving interest in pursuing a different line of synthetic chemistry as the diorganozinc chemistry was interesting, but was very specific in its application and could not be widely applied to different synthetic problems. It was during this time that A. J. Burke and coworkers had published an article in Tetrahedron: Asymmetry that inspired our group to explore the use of Ephedra derivatives as templates for making phosphine ligands. Thus, (1R,2S)-norephedrine and (1S,2S)-pseudonorephedrine were reacted with two equivalents of o-(diphenylphosphino)benzoic acid, two equivalents of 1-ethyl-3-dimethylaminopropyl carbodiimide hydrochloride (EDC-HCl), and a catalytic amount of DMAP to afford a series of phosphine ligands (Scheme 2). We learned that these easily prepared Ephedra derivatives could indeed serve as effective ligands in the palladium catalyzed asymmetric allylic alkylation of dimethyl malonate and afforded product enantioselectivities up to 88% ee. Our current efforts are now directed towards using the Ephedra alkaloids and other chiral, non-racemic beta-amino alcohols.
Scheme 2. Ephedra based o-(diphenylphosphino)benzoyloxy o-(diphenylphosphino)benzamide.
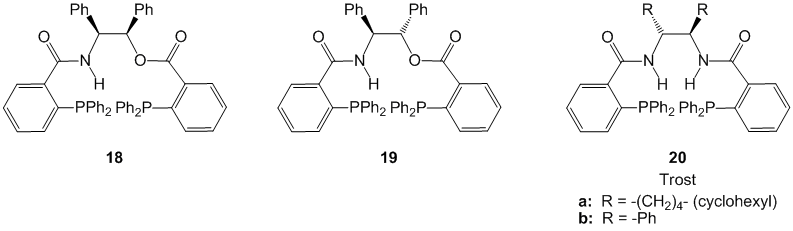
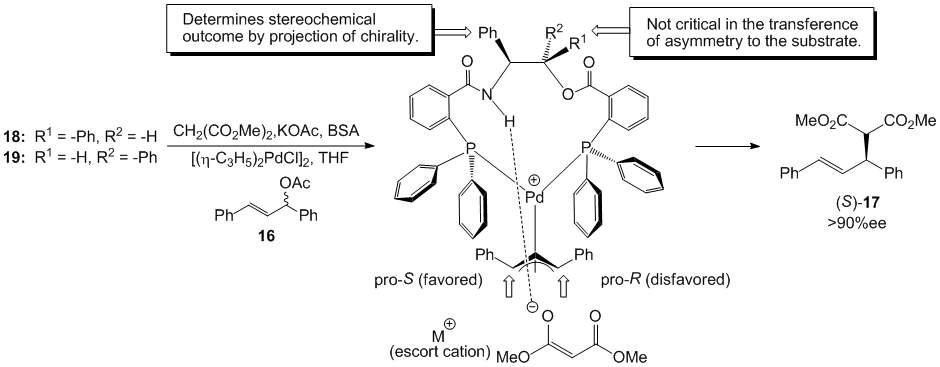
This work was followed by a more detailed study that involved the use of a number of beta-amino alcohols, both acyclic and cyclic, that yielded product enantioselectivities up to 95% ee. The best ligands from this body of work were based on the chiral scaffolds (1R,2S)- and (1S,2S)-2-amino-1,2-diphenyl-1-ethanol (Figure 2). These compounds are structurally related to the well known Trost ligand bis(amide) template but are b-amido esters rather than bis(amides).
Figure 2. Bis(diphenylphosphino) ligands developed by Hitchcock and coworkers.
When ligands 18 and 19 were employed in the asymmetric alkylation reaction with 1,3-diphenylpropenyl acetate 16 and the allylpalladium chloride dimer, each diastereomer generated the (S)-enantiomer of the product in enantiomeric excesses greater than 90% ee (Scheme 3). The ligands 18 and 19 afforded the same absolute stereochemistry in the product even though the two ligands are diastereomeric. In order to rationalize these observations, the working models of stereochemical induction pioneered by Trost and developed by Lloyd-Jones, Norrby, and coworkers, were employed. The working models ultimately suggested that the stereochemistry at the amido-position determined the stereochemical outcome of the asymmetric alkylation, and the stereochemical configuration at the alkoxy position was not instrumental in the transfer of asymmetry.
Scheme 3. Convergent stereochemical outcome from diastereomeric bis(phosphines) 18 and 19.
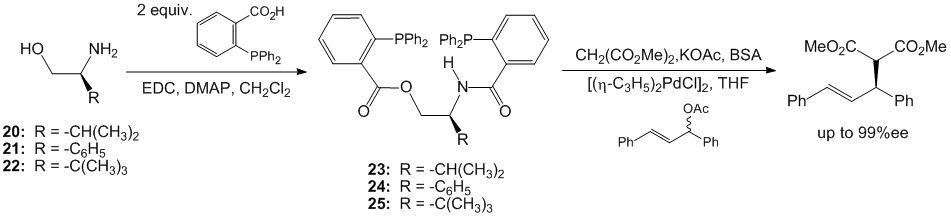
In an effort to further develop this research program, we synthesized a series of b-amido esters from commercially available L-valinol, L-phenylglycinol and L-tert-leucinol (Scheme 4). These derivatives were prepared in a single step by treatment of the beta-amino alcohols with two equivalents of o-(diphenylphosphino)benzoic acid and EDC with yields ranging from 56-71%. These ligands were applied in the benchmark standard catalytic reaction of allylic alkylation of dimethyl malonate with 1,3-diphenylpropenyl acetate. In conducting these reactions we learned that these easily prepared ligands afforded high enantioselectivies of the alkylation product in up to 99% enantiomeric excess! This preliminary work establishes a foundation for further development of this family of beta-amido ester bis(phosphine) ligands.
Scheme 4. Preliminary results on the synthesis and application of chiral, non-racemic b-amido esters.
Copyright © American Chemical Society