AmericanChemicalSociety.com
Reports: AC6 46671-AC6: Interactions Between Small Hydrocarbons and Water: Clathrate Formation and Inhibition
Mary Jane Shultz, Tufts University
The aim of this project is to determine the interactions involved in the nucleation of clathrates: On the molecular level, these substances consist of a guest molecule, often a hydrophobic one, surrounded by a cage of water molecules. The hydrophobic molecule concentration in the clathrates is typically on the order of 104 times larger than the corresponding solubility in water. One fascinating result is that the solid looks like ice, but it can support a flame. While burning snow is a novelty, the fundamental question is: “How does this huge change in concentration occur?” Previous experimental attempts to answer this question have mainly probed kinetics and thermodynamics associated with the growing clathrate crystal well after the nucleation process. Hence information about nucleation relied solely on theoretical models and calculations with scarce experimental data for verification. The goal of this project is to fill the gap in experimental data.
Our approach to this problem is to isolate water and the guest molecule, mainly hydrocarbons, in a solvent where water is sufficiently dispersed that it can be probed with infrared spectroscopy. We designed an optical cell capable of withstanding 100 atm. The optical aperture of the cell is 20 mm and can be equipped with sapphire, CaF2, or IR quartz windows depending on the spectroscopic and physical requirements of the experiment. It has been successfully used to 100 atm. – sufficiently high for all clathrates of interest.
To probe the initial interactions, we use carbon tetrachloride as a sort of ambient matrix isolation media. Wit was previously found that water exists in carbon tetrachloride as monomers. Further the dynamics of room temperature water have been modeled. The model of the dynamics is invaluable because it enables deconvolution of spectra with added solutes from that of residual water monomers to reveal the interaction dynamics between water and the solute. This strategy has been used for small inorganic salt solutes showing that salts enter the hydrophobic phase as ion-paired clusters and water associates with the cluster primarily via interaction between the water lone pair and the cation of the salt. The anion plays an important role in polarizing the water molecule. For the combination of a small cation and large polarizable anion, the result is formation of water-water hydrogen bonds. This is a much richer picture than the expected one of an ion surrounded by an intact hydration shell.
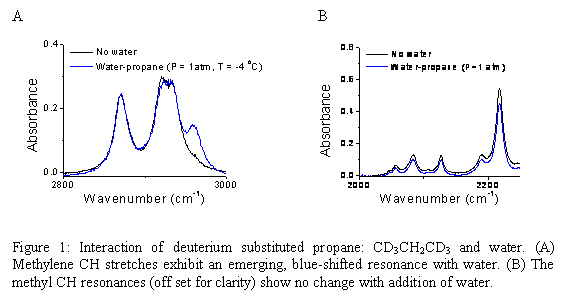
With the detailed model for water and water interaction with ionic solutes, this system was used to study the water-propane interaction. Preliminary results had suggested interaction between the methylene hydrogen atoms and the oxygen of water. To test this model, hexadeutero propane (deuterated at the methyl groups) was used. Figure 2 shows the results: The methylene resonances develop a new peak to the blue side of the methylene stretches while the methyl resonances are unaffected by water. As a side note: interaction between the lone pair of water and the methylene hydrogen has produced clear evidence of controversial blue-shifting hydrogen bonds.
A negative effect of clathrate formation is that the solid plugs gas pipelines, most famously with the recent gulf oil spill. It is thus of interest to understand how various inhibitors work. We have thus investigated methanol, one of the more widely used inhibitors. We find that methanol functions by competing for water in the propane-water-methanol system. There is no evidence for a direct methanol-propane interaction.
In summary, the water-carbon tetrachloride system has proven to be an excellent, near-room-temperature matrix-isolation system for probing interactions between water and various solutes including hydrocarbons typically found in clathrates.
1) Kuo, Margaret Hsinjui; David, Alexander; Kamelamela, Noelani; White, Mike; Shultz, Mary Jane, "Nitric Acid-Water Interaction Probed via Isolation in Carbon Tetrachloride" J. Phys. Chem. C 2007, 111, 8827-8831.
2) Kuo, Margaret; Kamelamela, Noelani; Shultz, Mary Jane, "Rotational Structure of Water in a Hydrophobic Environment: Carbon Tetrachloride" J. Phys. Chem. A 2008, 112, 1214-1218.
3) Bisson, Patrick; Xiao, Han; Kuo, Margaret; Kamelamela, Noelani; Shultz, Mary Jane, "Ions and Hydrogen Bonding in a Hydrophobic Environment: CCl4" J. Phys. Chem. A 2010, 114, 4051-4057.
4) Vu, Tuan; Kälin, Sarah; Shultz, Mary Jane, "Spectroscopic Identification of Water-Propane Interaction: Implications for Clathrate Nucleation" J. Phys. Chem. A 2010, 114, 6356-6360.
Copyright © American Chemical Society

