AmericanChemicalSociety.com
Reports: DNI7 49881-DNI7: Understanding the Diffusion of Polymers Confined between Interfaces
Christopher J. Ellison, PhD, University of Texas (Austin)
The dynamic behavior of polymers near interfaces is critical to performance in a broad range of applications including tertiary oil‐recovery from porous sediment, separation membranes [1] and intercalcation of nanocomposites [2, 3]. Unfortunately, the details of how interfaces impact dynamic molecular processes are still not well understood. In this research, the focus is on the longest possible dynamic length scale and studies the collective diffusion of polymer chains. The primary motivation for the study of interfacial diffusion is that the mechanisms and dependence of bulk polymer diffusion on fundamental variables, such as chain length and concentration, is well known for solutions and melts [4]. Understanding of bulk diffusion is crucial framework for developing an understanding of the parameters that govern interfacial diffusion (surface chemistry, size of confining dimension versus that of the polymer molecule, etc.) and for constructing theoretical models for predicting and describing the fundamental diffusion characteristics of polymers confined between interfaces.
In this research, fluorescence recovery after photobleaching (FRAP) is used to measure the diffusion coefficients of fluorescently labeled polymers confined between interfaces in thin films. FRAP is a well‐established technique for quantitatively tracking species diffusion in biology, but has not received significant attention in materials science [5‐8]. Figure 1a shows a cartoon schematic of a typical FRAP experiment where the fluorescent probe labeled polymer is spin coated onto a substrate and then high intensity light is used to irreversibly photobleach the fluorphores in a well‐defined area, but the polymer remains unchanged. Then lower intensity light is used to monitor the recovery of fluorescence back into the bleached region.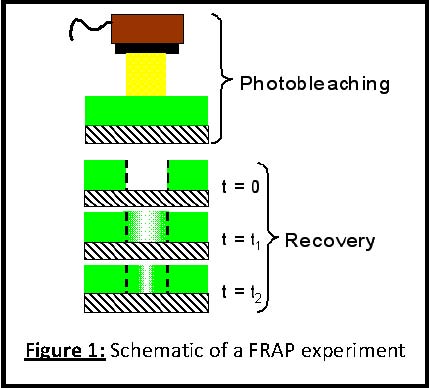
Using this analysis, the time profile of the fractional fluorescence intensity recovery can be fit to the solution of the diffusion equation (Eqn. 1) [9], assuming Fickian diffusion into a planar bleached region.
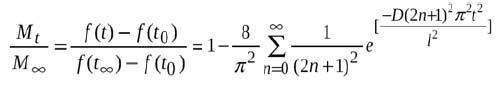
Mt is the total amount of diffusing species that has entered the bleached region from the reservoir up to time t and M∞ is the corresponding amount at infinite time. The ratio Mt/M∞ is equal to the fractional recovery of fluorescence intensity from time t=0 up to time t. This quantity is equal to the fluorescence intensity recovery at time t [f(t)‐f(t0)] divided by the total possible recovery up to t=∞ [f(t∞)‐f(t0)]. The parameter l is the length over which fluorescence recovery was measured that can be determined from the magnification of the image, t is the diffusion / fluorescence recovery time and D is the diffusion coefficient.
A7‐nitrobenzofurazan (NBD) fluorophore functionalized initiator was used to initiate an ATRP reaction to produce a fluorescently labeled poly(iso‐butyl methacrylate) (PiBMA). Gel permeation chromatography with fluorescence detection was used in our laboratory to verify the successful covalent attachment of NBD by confirming that the fluorescence signal peak sensitive only to NBD fluorescence with λexcitation = 418 nm, λemission = 565 nm) eluted at the same retention volume as the refractive index signal peak (sensitive to polymer concentration). This approach yielded polymer with a narrow molecular weight distribution (Mn = 10,400 g/mol and PDI = 1.06) in which every chain contained this fluorescence end group. The synthetic scheme used to produce this polymer is shown in Figure 2.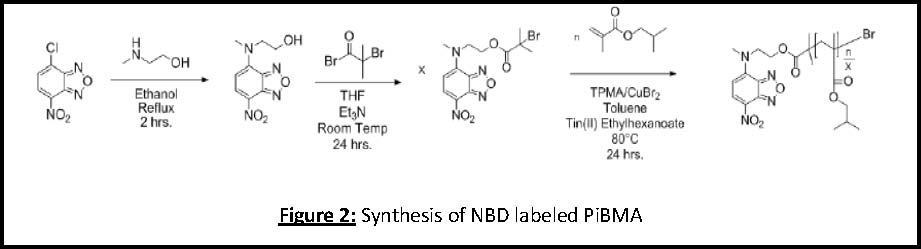
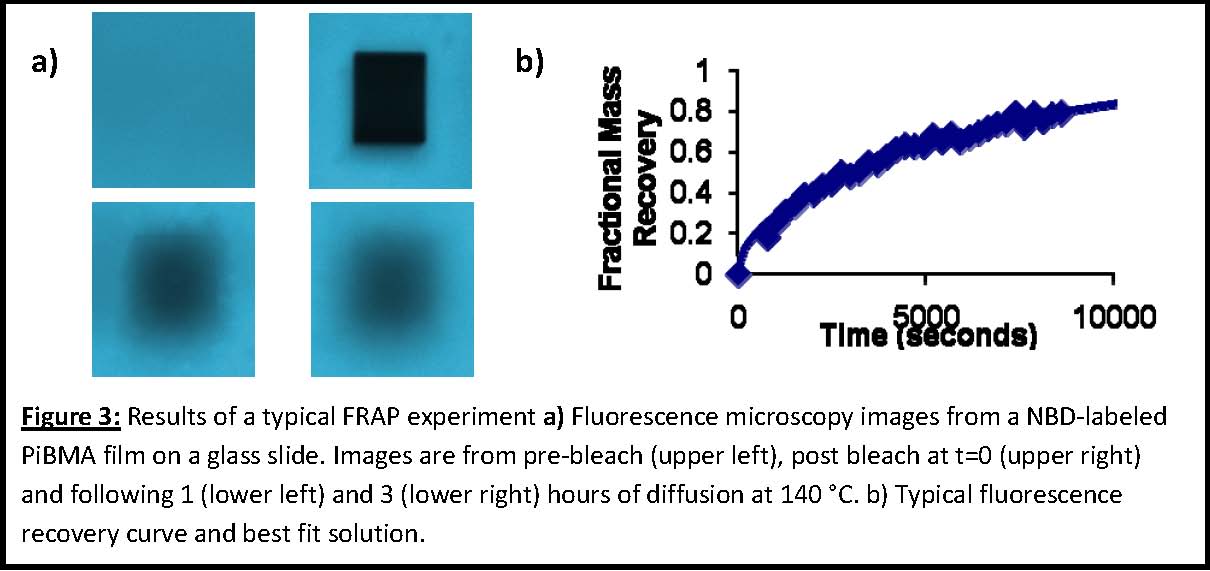
Figure 3a shows a series of images prebleach, postbleach, and during the recovery process using a 30 x 33 micron bleached region and the NBD‐labeled PiBMA sample synthesized from Approach 1 detailed above. The upper right image of Figure 3a has been bleached to 14% of its prebleach intensity. The superimposed black rectangle in the upper right panel is the region over which a left‐to‐right line integral of the intensity is performed (i.e., pixel intensity is averaged in the vertical direction and this value is a function of horizontal position) using ImageJ image analysis software (http://rsbweb.nih.gov/ij/). Figure 3b shows a typical recovery curve, the solid line is the best least squares fit to Equation 1.
The American Chemical Society – Petroleum Research Fund has provided for the startup of an entirely new project for the PI. This grant has been critical for preliminary experiments that have enabled proposals to be written encompassing a more intense, larger research effort to the National Science Foundation as a CAREER grant. This grant has supported one graduate student who is working on this project as the primary focus of his doctoral research. This was essential in his understanding of how to begin a research project from its beginning, which will benefit him in his future career. Using the travel allowance he was able to attend the American Chemical Society meeting in San Francisco and not only meet many of the leading names in the field for the first time, but also to get a better and broader picture of the state of polymer research.
- McCaig, M. S.; Paul, D. R., Effect of film thickness on the changes in gas permeability of a glassy polyarylate due to physical aging Part I. Experimental observations. Polymer 2000, 2.
- Schadler, L. S.; Kumar, S. K.; Benicewicz, B. C.; Lewis, S. L.; Harton, S. E., Designed interfaces in polymer nanocomposites: A fundamental viewpoint. MRS Bull. 2007, 4.
- Vaia, R. A.; Giannelis, E. P., Polymer nanocomposites: Status and opportunities. MRS Bull. 2001, 5.
- Granick, S.; Bae, S. C., Open questions about polymer interfacial diffusion. J. Polym. Sci. Pt. B‐Polym. Phys. 2006, 24.
- Axelrod, D.; Koppel, D. E.; Schlessinger, J.; Elson, E.; Webb, W. W., Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 1976, 9.
- Elson, E. L., Fluorescence correlation spectroscopy and photobleaching recovery. Annu. Rev. Phys. Chem. 1985.
- Sprague, B. L.; McNally, J. G., FRAP analysis of binding: proper and fitting. Trends Cell Biol. 2005, 2.
- Sprague, B. L.; Pego, R. L.; Stavreva, D. A.; McNally, J. G., Analysis of binding reactions by fluorescence recovery after photobleaching. Biophys. J. 2004, 6.
- Crank, J., Mathematics of Diffusion. 2nd ed.; Oxford University Press: New York, 1975.
Copyright © American Chemical Society

