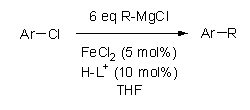AmericanChemicalSociety.com
Reports: B1 48541-B1: N-Heterocyclic Carbenes in Iron-Catalyzed Cross-Coupling Reactions
Marc C. Perry, PhD, Point Loma Nazarene University
A summary of the work that was proposed to take place this past year is given below:
1) Determine the conditions necessary to allow the cross-coupling of 2-chloropyridine and 1-hexyne.
2) Apply these conditions to the cross-coupling of other terminal alkynes and other aryl chlorides to test reaction scope.
Work left over from the previous year.
1) Complete a series of cross-couplings using unactivated aryl chlorides with primary and secondary alkyl Grignard reagents.
2) Complete the isolation and characterization of the new alkyl pyridine derivatives formed in the isonitile mediated cross-coupling of N-aryl chlorides.
Although more experiments can be attempted, initial studies into the cross-coupling of alkynes and aryl chlorides do not seem promising as no desired product was detected when 1-hexyne was reacted with 2-chloropyrimidine in the presence of FeCl2 and isonitrile or carbene ligand. The majority of work this past year was focused on the cross-couplings of unactivated aryl chlorides with primary and secondary alkyl Grignard reagents.
A series of cross-couplings involving three different unactivated aryl chlorides with primary and secondary alkylmagnesium chlorides were carried out using FeCl2 and a series of imidazolium chloride and imadazolinium tetrafluoroborate salts. The imidazolium and imidazolinium salts were converted into the corresponding imidazol-2-ylidines and imidazolin-2-ylidines in situ upon reaction with the strongly basic Grignard reagents. The different N-heterocyclic carbenes that were generated in situ and used in this study are shown in Scheme 1. The results of these cross-coupling reactions are shown in Table 1.
Scheme 1. NHC ligands used
The effect of ligand structure on the coupling of chlorobenzene
with butylmagnesium chloride was determined using the six different ligands
shown in Scheme 1 (entries 1-6). All of
the ligands resulted in some noticeable coupling, but in general, the
imidazol-2-ylidenes outperformed the imidazolin-2-ylidines, and the ligand 2, bearing the bulky diisopropylphenyl
groups, gave the best overall result (entry 2).
A comparison of entries 7 and 8 demonstrates that an increase in
Grignard reagent concentration increases the rate of the reaction indicating
that the Grignard reagent is either involved in the rate determining step of
the reaction, or alternatively serves a role in promoting the catalytic cycle
or preventing the catalyst decomposition.
Table 1. Entry L Ar R Time (h) Temp 0C % Yielda (B:L)b 1 1 Ph Bu 24 25 19 2 2 Ph Bu 24 25 80 3 3 Ph Bu 24 25 14 4 4 Ph Bu 24 25 24 5 5 Ph Bu 24 25 66 6 6 Ph Bu 24 25 12 7 2 Ph Prc 0.5 70 23 8 2 Ph Pr 0.5 70 56 9 2 Ph Pr 2 70 92 10 2 4-CH3Ph Pr 3 70 98 11 2 4-CH3OPh Pr 3 70 90 12 2 Ph Bu 1.5 70 98 13 2 4-CH3Ph Bu 3 70 80 14 2 4-CH3OPh Bu 3 70 75 15 2 Ph i-Bu 1 70 98 16 2 4-CH3Ph i-Bu 2 70 93 17 2 4-CH3OPh i-Bu 3 70 98 18 2 Ph Cy 3 70 56 19 2 Ph Cy 18 70 56 20 2 4-CH3Ph Cy 3 70 31 21 2 4-CH3OPh Cy 3 70 24 22 2 4-CH3OPh Cy 18 70 24 23 1 Ph sec-Bu 0.5 70 10 (16:1) 24 2 Ph sec-Bu 0.5 70 15 (9:1) 25 3 Ph sec-Bu 0.5 70 1 (4:1) 26 2 Ph sec-Bu 3 70 27 (5.8:1) 27 2 Ph i-Pr 3 70 33 (10:1) a) Yields determined by GC-MS using naphthalene as an
internal standard. b) branched to linear ratio c)
using 1.5 equivalents of the Grignard reagent The cross-coupling of a series of alkyl Grignard reagents
were then attempted with three unactivated aryl chlorides in THF at 70 0C
(entries 9-22). In general, primary
Grignard reagents coupled in excellent yields for all three unactivated aryl
chlorides including the challenging 4-chloroanisole. The secondary cyclohexyl Grignard gave
moderate to poor results with all three aryl chlorides. It was found that reaction times longer than
3 hours did not offer any improvement in the yields involving secondary alkyl
Grignards (entries 19 and 22).
The coupling of sec-butylmagnesium
chloride with chlorobenzene in THF at 70 0C was then attempted. The three imidazol-2-ylidene ligands that
were used in the earlier screen were employed to see if the same trends were
observed with the bulkier secondary alkyl Grignard reagents as seen in entries
23-25. Once again, the diisopropylphenyl
substituted ligand 2 produced the
best yield. Regardless of which ligand
was used, some of the n-butylbenzene isomer was observed with the mesityl
substituted carbene 1 giving the
best branched to linear ratio.
The diisopropylphenyl substituted ligand 2 was then employed in reactions
involving sec-butylmagnesium chloride
and isopropylmagnesium chloride as shown in entries 26 and 27. Although these reactions provided poor
yields, the branched to linear ratios were moderate. A comparison of entries 24 and 26 shows that
the branched to linear ratio decreased in the cross-coupling of sec-butylmagnesium chloride over time as
it went from 9:1 after 30 minutes to about 5:1 after 3 hours.
The current work was submitted to Tetrahedron Letters, and
the single reviewer felt it was not publishable in its current state. It was suggested that some isolated yields be
reported along a few other small changes.
At this time I would like to mention that I left my position
at the University of Alaska Anchorage in the middle of June, and took a
position at Point Loma Nazarene University in August. I did have some down time for research due to
this move.
The impact of this research was particularly significant for one
student that worked in my lab for two years.
Tyler Law made a decision to go on to graduate school in chemistry due
in large part to his experience performing research funded through this
proposal. I believe that he did not make
it into a program this year, but he will try next year.
Copyright © American Chemical Society