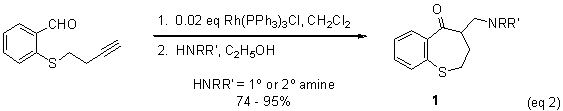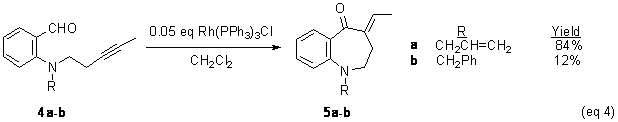AmericanChemicalSociety.com
Reports: UR1 49342-UR1: Rhodium(I)-Catalyzed Hydroacylation Promoted by Chelating Amines
Holly D. Bendorf, PhD, Lycoming College
Research in our laboratory is directed at developing synthetic routes to medium-ring heterocycles, a class of compounds that displays a broad range of biological activity. In particular, we are interested in exploiting the mild and selective rhodium-catalyzed hydroacylation as a strategy for the rapid construction of these ring systems.
Previously, we demonstrated that sulfur-containing 7- and 8-alkenals undergo intramolecular hydroacylation in the presence of Wilkinson's catalyst to yield cyclic sulfides (eq 1). The analogous 7- and 8-alkynals yield enone-containing heterocycles. While the reaction was rapid and high yielding for sulfides, the analogous ether and alkyl compounds (O or CH2 substituted for S) failed to cyclize, suggesting that coordination of the sulfur atom to rhodium was required for successful hydroacylation.
We have since applied this chemistry
to the synthesis of aminomethylbenzothiepinones, 1, which are intermediates in the synthesis of pharmaceutically
interesting compounds. Cyclization and
installation of the amino functionality are accomplished in one reaction vessel,
via a tandem hydroacylation-Michael addition sequence (eq 2). Most recently, we have applied our chelation-assisted
hydroacylation strategy to the synthesis of nitrogen-containing heterocycles. We have examined the reaction of several
2-(3-butenylamino)benzaldehyde substrates with Wilkinson's catalyst, Rh(PPh3)3Cl
(eq 3). Hydroacylation products are
obtained in each case; however yields of product depend on the identity of the
non-reacting amine substituent, R.
Significantly higher yields of hydroacylation product are obtained for
the allyl amine, 2a, suggesting that
the allyl substituent plays a key role in promoting hydroacylation, presumably
as a ligand on rhodium. A similar trend is observed for for the analogous alkynes, 4,although the rate of reaction is significantly
faster (eq 4). Hydroacylation of 4a was judged by GC to be complete
within 3 hours. In all cases, the only
significant product obtained was that due to hydroacylation. Products due to decarbonylation or rhodium-mediated
cleavage of the allyl group from the amine
were not observed. Recent and on-going work examines the scope and limitations
of the reaction with respect to the range of ring sizes accessible by this
chemistry and the tolerance of the reaction for substitution on the alkene,
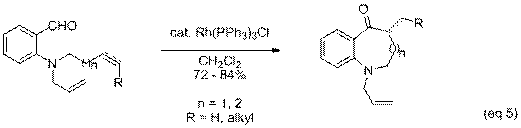
alkyne and carbon backbone (eq 5). We have
found that benzazocines (n = 2) can also be prepared via intramolecular
hydroacylation. Mono- and di-substituted
alkenes as well as internal and terminal alkynes smoothly undergo hydroacylation. However, tri-substituted alkenes fail to
react. Initial experiments with cationic
rhodium catalysts, such as [Rh(dppe)]BF4, have been successful and
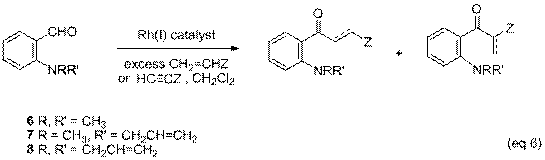
have produced results equal or superior to Wilkinson's catalyst. We have also investigated the intermolecular hydroacylation of
2-aminobenzaldehydes, 6-8. Initial experiments examined the reaction of
alkenes (methyl acrylate, styrene, 1,4-pentadiene) and alkynes (1-octyne) with
compounds 6-8, in the presence of
Wilkinson's catalyst or [Rh(dppe)]BF4. Only trace amounts of hydroacylation products
were observed in most cases. Work on this project has been completed by undergraduate
students at Lycoming College. Funds from
this grant supported two undergraduate research students during the summer of
2009 (Tess Duffin and Kyle Ruhl) and during the summer of 2010 (Robert Beamon
and Lauren Bottorf). The students prepared
a variety of allyl amine substrates and subjected these compounds to the
hydroacylation conditions. Tess also ran a number of intermolecular
hydroacylation experiments. Kyle Ruhl and
Carrie Harsomchuck were indirectly supported by this grant for their work
during the 2009-2010 academic year.
Carrie investigated the use of allyl amines as intermolecular
hydroacylation substrates. Kyle Ruhl
('11), Robert Beamon ('11) and Lauren Bottorf ('12) are chemistry majors at
Lycoming College. Carrie Harsomchuck graduated
with a B.A. in chemistry in May 2010 and is now enrolled in the Physician Assistant
program at Salus University. Tess Duffin
graduated in May 2010 with a B.S in chemistry.
Tess attends Villanova University and is pursuing graduate studies in
chemistry.
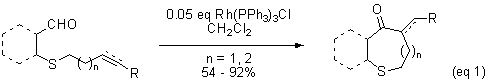
Copyright © American Chemical Society