AmericanChemicalSociety.com
Reports: AC1 41983-AC1: The Quest for Hydrocarbon Analogues of the Porphyrins
Timothy D. Lash, Illinois State University
Carbaporphyrins and related N-confused porphyrins (NCPs) has emerged as an important class of porphyrin analogues that exhibit intriguing chemistry and spectroscopic properties.1-3 These systems also readily form organometallic derivatives and may stabilize metal cations in relatively high oxidation states such as silver(III).3 Although porphyrin analogues with a single carbocyclic ring replacing one of the usual pyrrole units have been extensively studied over the last 15 years, very little progress has been made on the synthesis of dicarbaporphyrinoid systems. In recent investigations, we have developed three complementary routes to adj-dicarbaporphyrinoids and have made progress on the formation of macrocycles with three or four carbocyclic rings.
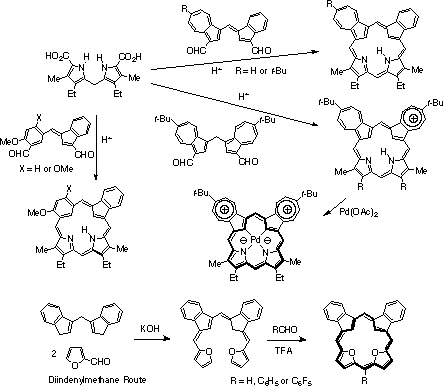
A general method for synthesizing fulvene aldehydes was developed by reacting an indene enamine with aromatic aldehydes in the presence of dibutylboron triflate.4-6 This methodology was used to prepare an azulene-fulvene dialdehyde that reacted with a dipyrrylmethane in the presence of TFA to give a 22-carbaazuliporphyrin.4 This new system shows significant diatropic character and undergoes an unusual oxidation reaction with silver acetate. The fulvene strategy was also used to prepare a 22-oxacarbaporphyrin using a furan derived fulvene dialdehyde.6 The adj-dicarbaporphyrin system with an indene unit adjacent to an azulene moiety proved to be far more stable than previously synthesized dicarbaporphyrins. This encouraged us to prepare fulvene dialdehydes with benzene derivatives and these were utilized to prepare dicarbaporphyrinoids with adjacent indene and benzene subunits.7 These 23-carbabenziporphyrins were also quite stable and exhibited diatropic character. Both dicarbaporphyrinoid systems underwent C-protonation onto the indene unit in the presence of excess TFA. In a second approach to dicarbaporphyrinoids, we investigated the synthesis of adj-diazuliporphyrins from a diformyl diazulenylmethane using a MacDonald '2 + 2' condensation. Excellent yields of mesoionic porphyrinoid products were obtained.8 These fully conjugated macrocycles showed significant diatropicity, and the internal CHs gave an upfield resonance near 0 ppm. These unusual porphyrinoids also afforded the corresponding palladium(II) complexes under mild conditions.8 The stable organometallic derivatives have two carbon-palladium bonds and must exist as a mesoionic structure. This unprecedented derivative was further characterized by X-ray crystallography, and takes on a near planar conformation.8 In a third approach, diindenylmethane was reacted with a series of aldehydes to give fully conjugated bilin analogues.9 The product derived from furfural was cyclized with paraformaldehyde, benzaldehyde or pentafluorobenzaldehyde to give aromatic dicarbaporphyrin analogues with two adjacent indene and two furan subunits.9 This approach provides a relatively direct route to porphyrin analogues and the ring closure step allows the introduction of diverse structural units. The synthesis of tri- and tetracarbaporphyrinoids has also been attempted, but aromatic macrocycles of this type have not yet been identified. Reaction of calix[4]azulenes with triphenylcarbenium salts afforded an analogue of the porphodimethenes, but a fully conjugated tetraazulene system could not be isolated.10 Nevertheless, these studies continue to produce intriguing results and new types of conjugated systems.5,10
References
1. Lash, T. D. in The Porphyrin Handbook, Ed. Kadish, K. M.; Smith, K. M.; Guilard,
R.; Academic Press: San Diego, CA, 2000,
Vol. 2, pp 125-199. 2. Lash, T. D. Eur. J. Org. Chem. 2007,
5461-5481. 3. Lash, T. D. Macroheterocycles 2008, 1, 9-20. 4. Lash, T. D.; Colby, D. A.; Idate,
A.; Davis, R. N. J. Am. Chem. Soc. 2007, 129, 13801-13802. 5. Davis, R. N. ; Lash, T. D. Tetrahedron 2009, 65, 9935-9943. 6. Jain, P.; Ferrence, G. M.; Lash, T.
D. J. Org. Chem.
2010, 75, 6563-6573. 7. Jain, P.; Lash, T. D. Unpublished
work. 8. Zhang, Z.; Ferrence, G. M.; Lash,
T. D. Org. Lett. 2009, 11,
101-104. 9. Lammer, A. D.; Ferrence, G. M.;
Lash, T. D. Unpublished work. 10. Lash, T. D.; El-Beck, J. A.; Colby, D. A. J. Org. Chem. 2009, 74,
8830-8833.
Copyright © American Chemical Society

