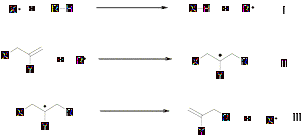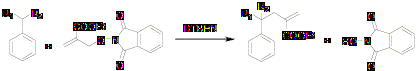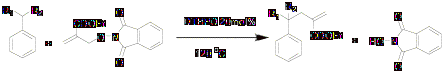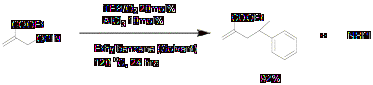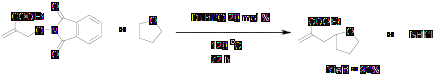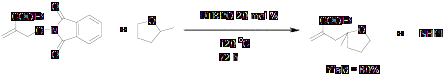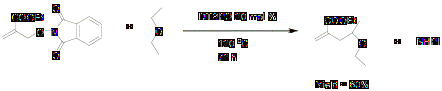AmericanChemicalSociety.com
Reports: AC4 48587-AC4: 'Radically' Green Approaches to Hydrocarbon Functionalization Via Allyl Transfer
James M. Tanko, Virginia Polytechnic Institute and State University
1. Introduction
Hydrocarbon functionalization via an allyl transfer reaction (Scheme 1, X = Br) using various allyl bromide substrates, has been studied in our group. Replacement of Br¥ by phthalimido-N-oxyl (PINO¥) was successful, and has helped make this chemistry environmentally benign. Reactions of allyl-phthalimido-N-oxyl (PINO) compounds for hydrocarbon functionalization have shown excellent results using high temperature initiators (di-tert-butylperoxide) and reactions are under investigation with respect to low temperature initiators (triethylborane, di-tert-bytylhyponitrite). Similarly, functionalization of ethers (tetrahydrofuran, 2-methyltetrahydrofuran, dioxane, diethyl ether etc.) has shown interesting results and is being studied further.
Scheme 1
The advantage of PINO¥ chemistry is the byproduct of the reaction, NHPI (N-hydroxyphthalimide), is easily recovered and separated as a while solid at the end of the reaction. We synthesized "Allyl-PINO" substrates using several published methods1. Although, we could not completely eliminate the use of allyl bromides from these synthetic pathways, our preliminary objective is to study PINO¥ chemistry in hydrocarbon functionalization and then we can focus more on an improved synthesis of "Allyl-PINO" substrates.
Earlier we reported that PINO chemistry works well with initiator DTBPO (di-tert-butylperoxide, a convenient source of t-butoxyl radical) leading to excellent yields. Also, we have reported the comparison between Br¥ and PINO¥ concluding that PINO¥ is more selective than Br¥ thus, PINO chemistry leads to high mass balance and better yields compared to Br¥ chemistry.
2. Experiments and Results
2.1 Di-tert-butylhyponitrite (DTBHN) as a radical initiator
DTBHN can be used in initiation processes at fairly low temperatures; like DTBPO, DTBHN is a source of t-butoxyl radical. In order to minimize the temperature requirements we chose DTBHN as an initiator for these reactions. We synthesized the initiator using tert-butyl bromide and sodium hyponitrite in ca. 60% yield.
Scheme 2
Hydrocarbon functionalization via allyl transfer was performed using DTBHN as an initiator as follows.
Scheme 3
The results of this free radical reaction using di-tert-butyl hyponitrite as an initiator (Scheme 3) are summarized in the Table 1.Yield and mass balance is low at room temperature. Reaction shows maximum yield at 65 oC with high mass balance.
Entry |
R1 |
R2 |
Temperature (°C) |
Time (h) |
Yield (%) |
PINO compound left (%) |
Mass balance (%) |
1 |
H |
H |
r.t. |
96 |
31 |
41 |
72 |
2 |
H |
CH3 |
r.t. |
96 |
36 |
39 |
75 |
3 |
CH3 |
CH3 |
r.t. |
168 |
23 |
45 |
68 |
4 |
CH3 |
H |
65 |
12 |
65 |
35 |
100 |
Table 1
Initiator used was 20mol% and hydrocarbon was used as a solvent.
2.2 Kinetic chain length measurements
Kinetic chain lengths were measured for the reaction (scheme 3) and summarized in table 2
Initial chain length | |
R1 = R2 = H | R1 = H, R2 = CH3 |
1.35 | 1.86 |
Table 2
As the decomposition rate constant of the initiator at 45°C has been reported, we decided to perform the chain lengths experiments at 45°C. Above results above show that chain length is around 1 which means that this, is not a chain reaction and probably DTBHN is not the best initiator for these reactions.
We also calculated chain lengths of the reactions in which we used DTBPO as an initiator at higher temperature. The results were satisfactory as expected.
Scheme 4
Results are summarized in table 3
Initial chain length | ||
R1=R2=H | R1=H, R2=CH3 | R1=R2+CH3 |
266 | 129 | 11.5 |
Table 3
2.3 Triethylborane/O2 as a radical initiator
Organoboranes have been used, as a radical initiator, in many transformations. Our idea was to use triethylborane/O2 to initiate radical formation in allyl transfer reactions. Triethylborane is an effective initiator even at lower temperatures (-78 o C), which may lead to improved regio and stereoselectivity.
Hydrocarbon | TEB equiv. | Temp. oC | Time | yield %
|
Toluene | 0.2 | 0 | 24 | N/O |
Toluene | 0.2 | RT | 24 | 1 |
Toluene | 0.5 | RT | 24 | 2 |
Toluene | 0.5 | 50 | 12 | 10 |
Toluene | 0.5 | 80 | 12 | 30 |
Cumene | 0.5 | 80 | 12 | 34 |
Ethylbenzene | 0.5 | 80 | 12 | 32 |
Table 4
Table 4 shows the summary of results, all performed on α-bromo methyl styrene. The same reactions were performed on "Allyl-PINO" substrates at 80 oC for 12 hrs (table 5 ). In entries 1-6 we used α-PINO methyl styrene and in entries 7-9 we used α-PINO methyl acrylate Yields were just slightly improved (by 3-4%).There was no dramatic difference in yields. Our mass spectra analysis confirmed that, 10%-15% of the reaction leads to the addition of ethyl radical to the reactive double bond of "Allyl-PINO" substrates. Although that does not completely explain low yields of these reactions, we think that this might have happened because,
á Either TEB, a precursor of reactive ethyl radical can lead to side reactions, and thus, is not a good initiator or selective H-abstractor.
á The radical addition step is slow at low temperatures. (according to our results any temp. lower than 120 oC is lower for these reactions.)
Hydrocarbon | TEB/O2 (equiv) | Temp. oC | Time h | yield % |
Toluene | 0.5 | 0 | 12 | N/O |
Toluene | 0.5 | rt | 24 | 5 |
Toluene | 0.5 | 50 | 12 /24 | 35 |
Toluene | 0.5 | 80 | 12 | 40 |
Cumene | 0.5 | 80 | 12 | 45 |
Ethylbenzene | 0.5 | 80 | 12 | 42 |
Toluene | 0.5 | 80 | 12 | 36 |
Cumene | 0.5 | 80 | 12 | 40 |
Ethyl benzene | 0.5 | 80 | 12 | 36 |
Table 5
2.4 Lewis acid catalyzed allyl transfer reactions
We decided to use Lewis acids in these reactions because, if addition of the C-centered radical is the problem step that should be resolved by lewis acid catalysis, making "Allyl-PINO" substrates more electrophilic. Reaction was successful with excellent yield.
Scheme 5
2.5 Ether functionalization via allyl transfer reactions
Ether functionalization is very rare in organic synthesis. Existing methods consist use of toxic organometallic reagents. Since, ethers like THF, Me-THF, dioxane etc. has weak C-H bonds, our idea was to treat them as we did hydrocarbon and carry out allyl transfer reactions. We are currently working on ether the functionalizations via allyl transfer reactions and we have obtained some very interesting results.
Scheme 6
Copyright © American Chemical Society