AmericanChemicalSociety.com
Reports: UR1 50086-UR1: Oxidation of Alkenes in Aqueous Solvent Mixtures Using Environmentally Benign Reagents
Thottumkara K. Vinod, Western Illinois University
The central theme and the overarching goal of the currently funded proposal is the development of eco-friendly and green protocols for oxidative transformations of alkenes. In this report for year 1 we summarize our preliminary but significant results of the two projects that my students and I engaged in during the last year. These two successful projects were directed at (a) using benign hypervalent iodine reagents as ‘ozone' equivalents for oxidative cleavage of alkenes and the (b) development of a convenient and iodine atom economic co-iodination protocol for alkenes. All student participants in the projects received extensive training in wet chemistry, chromatographic techniques as well as spectroscopic methods.
In keeping with
green trends in organic synthesis, Ochiai et. al. recently introduced environmentally
benign organoiodine reagents [PhIO, 48%HBF4, 18-Crown-6 and
ArI (cat), 48% HBF4, mCPBA] for the oxidative cleavage
of alkenes. Continuing our interest in developing catalytic and selective
oxidation protocols using water-soluble hypervalent iodine reagents in
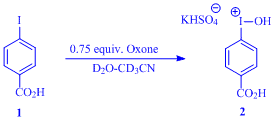
presence of Oxone as a co-oxidant, we reasoned that the oxidation of 4-iodobenzoic
acid (4-IBAcid, 1) with Oxone would yield [hydroxy(4-carboxyphenyl)iodonium]ion,
2,
a structural derivative of the active reagent reported by Ochiai et. al.
for alkene functionalization Herein, we report a facile
and operationally convenient protocol for the oxidative cleavage of alkenes
and vicinal diols in aqueous acetonitrile using catalytic amounts of 4-IBAcid
in presence of Oxone as a terminal oxidant. Our initial efforts were directed
at establishing the easy oxidation of 1 with Oxone in aqueous acetonitrile
to produce the corresponding iodonium ion, 2, the desired active
reagent. Treatment of 1 with 0.75 equiv. of Oxone in D2O:CD3CN
(3:1 v/v) readily provided 2 and subsequently we set out to investigate
the utility of 2 as an in situ generated reagent for oxidative
cleavage of alkenes.
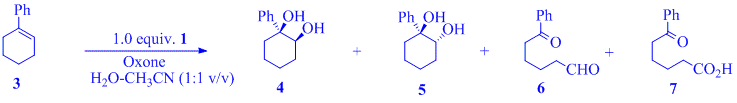
Commerically available 1-phenyl-1- cyclohexene 3 was chosen as the test substrate upon which optimization studies were performed. Our initial attention was focussed on determining whether Oxone alone promoted alkene cleavage. Entries 1 and 2 of Table 1 demonstrate that while Oxone does convert alkene 3 to the diol products 4 and 5, no oxidative cleavage is observed. However, in the presence of 4-IBAcid, alkene 3 is cleaved to the corresponding oxidized products 6 and 7. The yield of keto-aldehyde 6 and keto-acid 7 is dependent on the ratio of 4-IBAcid to Oxone. Entries 3-9 demonstrate that in the presence of 1.0 equiv. of 1, varying equivalents of Oxone results in changes in product distribution; an increase in Oxone concentration is met with a parallel increase in formation of cleaved products 6 and 7. The yield of 6 is never high due to rapid aldehyde oxidation to 7 by Oxone. We identified that 1.5 - 2.0 equiv. Oxone proved sufficient for complete conversion of 3, (a trisubstituted alkene) to 7 (a keto-acid). The isolated product mixture from this optimization study contained small (<20%) amounts of 1indicating that the major bulk of the stoichiometric amount of 1 initially employed in these reaction was lost during the work-up either as the water-soluble iodonium ion, 2, or through the polymerization of the iodosyl derivative. Irrespective of how the loss of 1 occurred, the observed quantitative cleavage of 3 with only sub-stoichiometric amounts of 1 indicated the feasibility of a catalytic protocol which we verified by using sub-stoichiometric quantities of 1 along with 2.0 equiv of Oxone to obtain near quantitative clevage of 3 (entries 10-12).
| entry | equiv. 1 | equiv. Oxone | % yield 4+5 | % yield 6 | %yield 7 | entry | equiv. 1 | equiv. Oxone | %yield 4+5 | % yield 6 | % yield 7 |
| 1 | 0.0 | 0.5 | 95 | -- | -- | 7 | 1.0 | 1.5 | 8 | -- | 92 |
| 2 | 0.0 | 1.0 | 100 | -- | -- | 8 | 1.0 | 1.63 | 3 | 5 | 92 |
| 3 | 1.0 | 0.5 | 95 | 5 | -- | 9 | 1.0 | 2.0 | -- | -- | 100 |
| 4 | 1.0 | 0.75 | 69 | 7 | 24 | 10 | 0.5 | 2.0 | -- | -- | 100 |
| 5 | 1.0 | 1.0 | 45 | 15 | 40 | 11 | 0.25 | 2.0 | -- | -- | 100 |
| 6 | 1.0 | 1.2 | 25 | 10 | 65 | 12 | 0.05 | 2.0 | -- | -- | 100 |
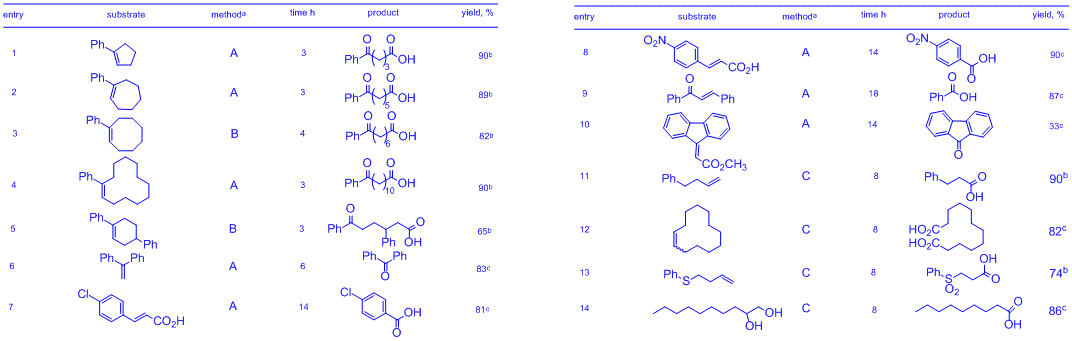
These optimization studies with stoichiometric 1 served as a proof of principle that our in situ generated oxidant 2 effectively cleaves alkenes in the presence of Oxone. The ability to use 1 catalytically, in concert with the operational simplicity of this reaction, prompted us to investigate the scope of this transformation. Table below shows a selection of alkenes cleaved under the newly developed conditions.
In summary we have developed an operationally simple and a catlaytic procedure for oxidative cleavage of alkenes using benign and cheap reagents.
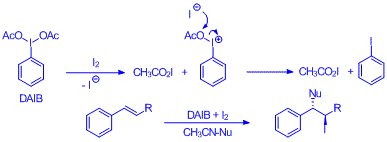
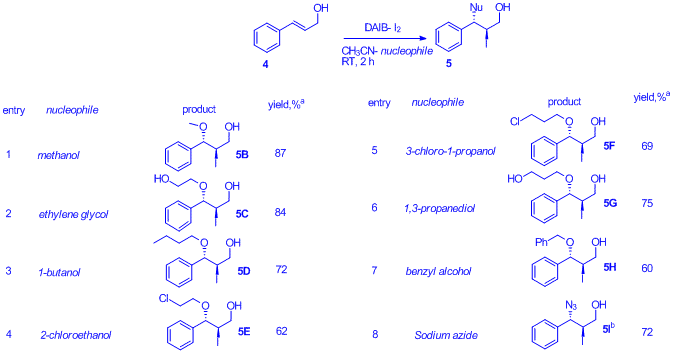
Molecular iodine, I2, is readily converted into 2 equiv. of acetyl hypoiodite (CH3CO2I) via oxidation by (diacetoxyiodo)benzene (DAIB) followed by the trapping of the iodide ion by acetoxyphenyl iodonium ion formed. The in-situ generated CH3CO2I is utilized for the synthesis of 1, 2-iodo-cofunctionalized derivatives of a variety of alkenes. Conversion of both iodine atoms of I2 to I+ sources results in 100% iodine atom economy for the reported iodo-cofunctionalization of alkenes
The table below shows a variety of co-iodination derivatives of cinnalyl alcohol prepared using the described methadology. We are hopeful that a variety of alkenes can be co-iodinated using this procedure and a broad substrate scope investigation is one of the immediate goals of this area.
In conclusion, we believe that significant progress has been made in both areas of investigation and hope to relay our continued success in these areas to PRF through publications and annual reports.
Copyright © American Chemical Society