AmericanChemicalSociety.com
Reports: DNI1 49279-DNI1: Development of Intramolecular [4+1] Cycloadditions
Tristan H. Lambert, PhD, Columbia University
This program is aimed at developing new formal [4+1] cycloaddition reactions for the synthesis of complex five-membered rings. The research conducted with financial support from the PRF has lead to the invention of a novel and potentially broadly useful [4+1] strategy and, in addition, to the discovery of several related reaction manifolds that we expect will be of significant synthetic utility.
Our proposed studies were based on our initial report of a new [4+1] strategy that involved the direct palladium(II)-catalyzed intramolecular cyclopropanation of 1,3-dienes by pendant beta-ketoesters, followed by a mild vinylcyclopropane-cyclopentene (VCP-CP) rearrangement mediated by magnesium(II) iodide. One of the significant limitations of this technology was the need for a gamma- or delta- quaternary carbon to block undesired Saegusa-type oxidation of the substrate by the palladium catalyst. Because we had observed a significant effect of the palladium carboxylate ligands in terms of reaction efficiency, our initial efforts were aimed at investigating the role of these ligands with the goal of suppressing the undesired oxidation. Unfortunately, no clear steric or electronic trends were observed from this study, nor was any further optimization achieved.
Therefore, we decided to pursue an alternative strategy, which has resulted in the development of an iodine/magnesium salt mediated [4+1] methodology that does not require a blocking functionality, works in a single flask, is highly efficient, and utilizes simple and inexpensive reagents rather than transition metals. This approach was inspired by the fact that the first step of our original work required the use of a magnesium(II) Lewis acid (presumably to promote keto-enol tautomerization), and the second step utilized magnesium(II) iodide. We reasoned that if the oxidative cyclopropanation could be effected with the magnesium Lewis acid and molecular iodine as the oxidant instead of palladium, that this process would produce magnesium iodide in situ and thus provide for a one-pot [4+1] process. In fact we have found this strategy to be quite effective, allowing for formal [4+1] reactions even with unblocked 1,3-dienyl beta-ketoesters (eq. 1). We are currently completing our substrate scope studies and a manuscript describing this work is in preparation.
In addition to this work, we have found that palladium(II)
catalysis allows for the facile [3+2] cycloaddition of beta-ketoesters and
1,3-dienes to form 2-vinyl dihydrofurans.
Interestingly, this chemistry works in both intra- and intermolecular
settings, and may prove to be a useful new tool for the preparation of these
important heterocycles.
In further work, although the Saegusa-type oxidation we
observed in our original method development was undesired, we recognized that
in other contexts such reactivity can be highly advantageous. We thus decided to investigate whether
this direct oxidation could be generalized to other carbonyl compounds. In fact, we have found that aliphatic
aldehydes, in the presence of magnesium(II) perchlorate, are prone to
palladium(II) catalyzed oxidation to produce unsaturated aldehydes. We believe this method may be of
significant utility in a range of contexts, and we are preparing a manuscript
to relate these results.
The funding provided by the PRF has allowed us to advance
our [4+1] program with the development of a highly efficient strategy, which we
believe will prove very effective for the construction of a range of important
five membered ring architectures.
Our work has also uncovered several findings in regard to palladium
oxidation chemistry, which have provided us with new avenues of research to
pursue. In part due to the work
supported by this grant, our group was able to successfully compete for an NSF
CAREER grant. Our continuing
efforts to advance these initial findings, including the further development
and application of our novel [4+1] methodologies, serves as one of the
cornerstones of our group's research program.
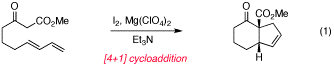
Copyright © American Chemical Society

