AmericanChemicalSociety.com
Reports: G1 48479-G1: Synthesis of Chiral Allenyl Ethers: Memory of Chirality in the Cyclization Reactions of Oxyallyl Cations
Corey R. J. Stephenson, Boston University
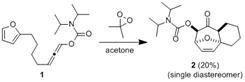
In the second year of funding from the Petroleum Research Fund, we aimed to improve the cycloaddition chemistry discovered during the first year and generalize the process for use in the preparation of complex molecules (ie. 1 → 2). Unfortunately, attempts to improve the efficiency of this, and related transformations, were unsuccessful.
At
about the same time, my research group was focusing upon the development
of new methods utilizing visible light
activated photocatalysts for organic synthesis. Using support from the
Petroleum Research Fund, we have published 2 manuscripts during the past year,
with two additional manuscripts currently under review. On the
basis of observations made during the early stages of our research in this area,
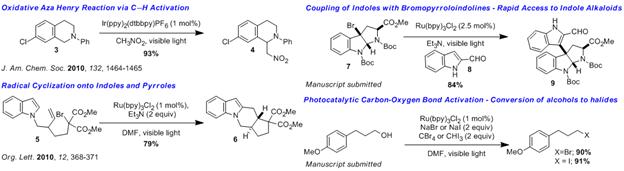
we were able to develop an oxidative aza-Henry
reaction using photoredox catalysis [J.
Am. Chem. Soc. 2010, 132, 1464]. This extraordinarily
straightforward method was performed by simply dissolving the desired tertiary
amine 3 in nitromethane with 1 mol %
of known Ir catalyst and irradiating with a hand held compact fluorescent light
source for up to 18 h, providing 4
in excellent yield. We were able to expand the utility of photoredox catalysis
as a means to initiate radical reactions with the development of a new
methodology for the cyclization of a-bromomalonates
and imides onto indoles and pyrroles [Org.
Lett. 2010, 12, 368]. In particular, this mild, catalytic method (1 mol% Ru) efficiently
converted affected a cascade radical cyclization of 5, providing the tetracyclic product 6 in 79% yield as a single diastereoisomer. We have recently
developed intermolecular radical coupling strategies which provide rapid access
to indole alkaloid scaffolds from readily available bromopyrroloindolines such
as 7. The coupling of 7 with 8 in the presence of 2.5 mol % of Ru catalyst provided the desired
coupling product, 9, in 84% isolated
yield. Available in only 3 steps from
commercially available material, 9
is expected to be the common intermediate in the synthesis of the bisindole
alkaloid family of natural products. Finally, we have developed a catalytic
variant of the Appel reaction using photoredox catalysis. The stoichiometric
reductant Ph3P can be replaced by 1 mol % of the Ru catalyst and
visible light irradiation, providing bromides or iodides in high chemical yield
under mild reaction conditions. This method constitutes a new approach to
catalytic carbon-oxygen bond activation which we are currently investigating
for nucleophilic displacement chemistry.
Copyright © American Chemical Society