AmericanChemicalSociety.com
Reports: ND4 48907-ND4: Monovalent, Ultra Long-Lived Fluorescent Organic Nanoparticles
Steven Zimmerman, PhD, University of Illinois (Urbana-Champaign)
Polyglycerol dendrimers and hyperbranched polymers (HPGs) have a number of attractive properties that have led to their use in a broad range of applications from drug delivery devices to catalysis to diversity platforms for synthesis. Arguably, the most important property is the biocompatibility afforded by the water-soluble, highly branched polyether-polyol structure. HPGs resist protein adsorption and show toxicity profiles in cell studies that are at least as good as polyethylene glycol (PEG). Beyond their biocompatibility, HPGs can be prepared on kilogram scale in one step from commercial reagents. With funding from the ACS PRF, we have been exploring the use of HPGs to stabilize fluorescent dyes.
The favourable features of HPGs described above had led to considerable effort to specifically functionalize the HPG core. For example, 8 step routes to azide and alkyne-cored polyglycerol dendrons and their subsequent click reaction were recently reported. Herein we report the straight forward and scalable preparation of alkyne-cored HPG. Both high (>50,000 Mn) and low (<10,000 Mn) molecular weight polymers could be accessed with higher Mn material synthesized using an emulsion polymerization method. The utility of the low Mn HPGs was shown by clicking them to fluorescein, biotin, as well as their use in stabilizing gold nanoparticles (NP) and in a pH-sensitive nanocarrier to catch and release small molecules.
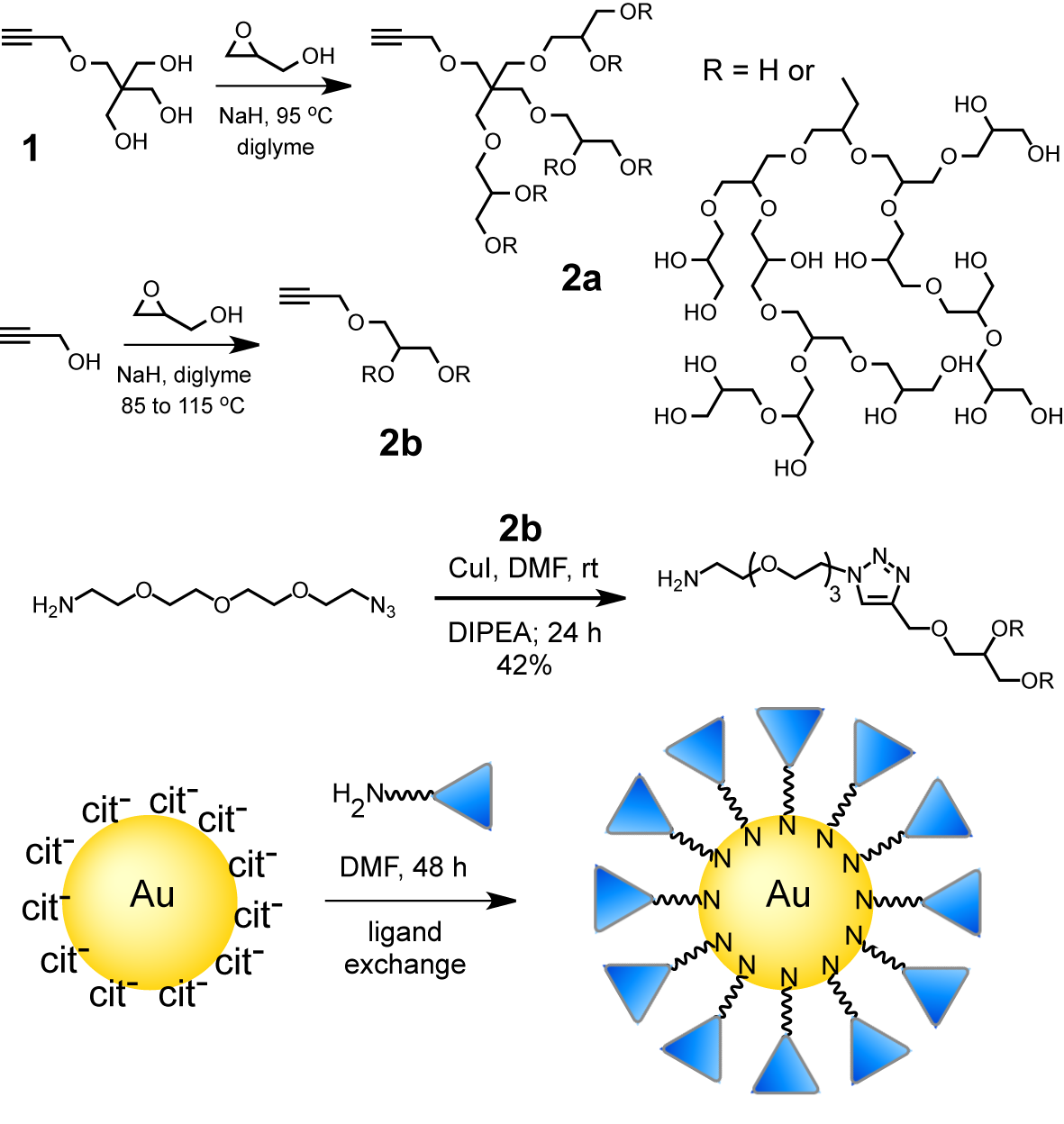
Two routes to alkyne-functionalized HPG are outlined in Figure 1. The first uses triol initiator 1, prepared in three steps from pentaerythritol. Thus, a diglyme solution of 1 was treated with 10 mol % sodium hydride and glycidol was slowly added to produce HPG 2a. Typical Mn and PDI values (Mw/Mn) for purified polymers are 7,000 to 14,000 and 1.3 to 1.5, respectively. An alternative synthesis uses propargyl alcohol as an alkyne source allowing the HPG to be prepared in a single step. The precipitation process used to purify the polymers reduced the PDI and increased molecular weights by removing lower molecular weight polymer. This process was further refined and used to fractionate polymer 2b giving material with a lower PDI and 77% mass recovery overall. The presence of the propargyl group was confirmed using MALDI and NMR. Inverse gated 13C NMR was used to confirm the branched structure of HPG 2b and indicated a degree of branching of 58%.
By virtue of the single alkyne group at the focal point, these HPGs can be covalently linked to a broad range of compounds and materials using standard click chemistry. To illustrate this potential in a chemical biology context, 2b was reacted with a biotin azide and also clicked to a fluorescein azide giving a fluorescent PG.
Because of their biocompatibility and resistance to protein adsorption, it is expected that HPG 2b will also be useful as a nanoparticle (NP) based surface coating. Rather than linking 2b to the NP by a click reaction, an amine functionalized HPG was synthesized by clicking to an amino azide. The amino-tipped HPG was used to coat 13.5 ± 1.1 nm diameter, citrate-capped gold NPs, prepared using the Frens method. This resulted in the formation of stable HPG-capped gold NPs that could be isolated without aggregation. These particles could be spun down and resuspended in DI water.
As with NPs, drug delivery agents such as unimolecular micelles rely on a biocompatible surface coating for extended circulation in the body and for minimizing toxicity. Recently we reported a method for the synthesis of degradable dendrimers and hydrogels utilizing 1,3,5-triazaadamantane (TAA) monomers. These hydrophobic dendrimers are acid-labile but lack water solubility. Because they are built through a series of click reactions, they present an excellent synthetic scaffold for the synthesis of acid-sensitive unimolecular micelles using alkyne-functionalized HPGs.
A second
generation TAA dendrimer (MW = 11,402; 27 azide groups) was used as the core
and was reacted with one equivalent of HPG 2b (fraction 3, Mn
= 3000, PDI = 1.25). The resulting
polymer was fully water-soluble and exhibited a narrow molecular weight range (Mw/Mn
< 1.15). DLS measurements of concentrated solutions of the polymer (2.5 mg/mL)
indicated it to be monomeric in phosphate buffer with an average hydrodynamic
diameter of 8.4 nm. In addition,
an MTT cell viability assay showed that the polymer is well-tolerated by cells
at concentrations up to 0.75 mg/mL with a toxicity profile similar to HPG
alone. Titration of
1-anilino-8-sulfonic acid (1,8-ANS) was used to verify that the polymer could encapsulate
non-polar guest molecules. The 1,8-ANS probe was bound by the polymer,
exhibiting a 1:1 binding isotherm with an apparent Ka =
2.0 x 104 M-1. The enhanced fluorescence observed for encapsulated
1,8-ANS also provides a convenient method of monitoring dendrimer
degradation. Thus, the
fluorescence of 1,8-ANS at various pH values was monitored during the addition
of 2.4 equivalents of the polymer.
Time-dependent fluorescence plots show a spike with polymer addition as
a result of the uptake of the dye into the hydrophobic interior. This is followed by a first order decay
of the fluorescence as the dendrimer degrades and the dye is released back into
solution. The decay is pH
dependent with steady state fluorescence reached in 5 min at pH 1.7 and over 4
h at pH 4. Thus
far, we have developed a simple procedure for the synthesis of alkyne
functionalized HPG. These polymers
facilitated the preparation of potential biological imaging agents such as
monofunctionalized fluorescein and biotin HPG, the stabilization of gold
nanoparticles, and the synthesis of unimolecular acid-labile micelles.
Copyright © American Chemical Society

