AmericanChemicalSociety.com
Reports: AC1 48199-AC1: New Synthetic Methods Involving Wittig Rearrangement Processes
John P. Wolfe, University of Michigan
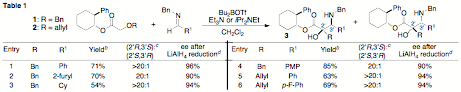
During the past year we have developed a new asymmetric tandem Wittig rearrangement/Mannich reaction as described in our original proposal. Asymmetric induction was achieved using a simple chiral auxiliary (2-phenylcyclohexanol), which is commercially available. As illustrated in Table 1, transformations of a number of amino alcohols 3 are generated in good yield with good to excellent stereoselectivity when N-benzyl or N-aryl imines are employed as electrophiles. Reduction of the products with LiAlH4 affords enantiomerically enriched aminodiols with up to 96% ee.
a Conditions: 1.0 equiv 1 or 2, 1.5–2 equiv R1C(H)NR2,
3.2 equiv Bu2BOTf, 4 equiv Et3N (R = Bn) or iPrNEt2 (R = allyl), CH2Cl2,
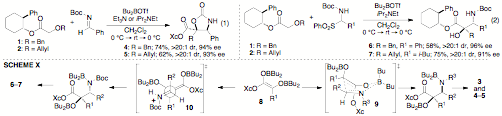
0°C → rt → 0°C. b Isolated yield (average of two or more experiments). Use of N-Boc imines affords trisubstituted
isoxazolidin-2-ones 4–5 derived from syn-aminoalcohols in moderate yield with
excellent stereocontrol (eq 1). In contrast, use of 2-(phenylsulfonyl)amines as
electrophiles affords anti-aminoalcohol
products 6–7 with
high dr (eq 2). In all cases highly enantiomerically enriched aminodiols are
obtained (up to 96% ee) after reduction of the products with LiAlH4.
The carbamate functionality is not affected during the reduction; selective
reduction of the ester is observed. The relative
stereochemistry of the amino-alcohol product is set during the subsequent
Mannich reaction, and is dependent on the nature of the electrophile. In
reactions involving N-benzyl or N-Boc imine electrophiles the Mannich reactions occur
via boat-like transition state 9 to afford the syn-amino alcohol products 3 and the
oxazolidin-2-ones 4–5 (Scheme 1). In contrast, reactions of N-Boc-2-(phenylsulfonyl)amines
likely involve intermediate N-Boc
iminium ions. The Mannich reactions of the iminium-ions occur via open
transition states 10 in which R2 is positioned away
from the two bulky boron groups. This gives rise to the observed anti-amino alcohol products 6–7. We have also
made two significant advances in the area of tandem Wittig Rearrangement/Aldol
Reactions. We have successfully generated vicinal quaternary stereocenters with
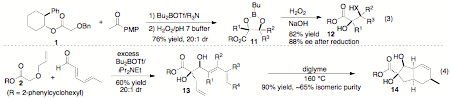
high stereocontrol through the reaction between O-benzyl-2-phenylcyclohexyl glycolate ester 1 and p-methoxyacetophenone to afford boronate ester 11
in 76% yield as a single stereoisomer (eq 3). Subsequent cleavage of the
boronate ester gave 12 in 82% yield
(assayed as 88 % ee after subsequent auxiliary cleavage). We have also employed
the tandem Wittig rearrangement/Aldol reaction sequence to generate 13, which undergoes an intramolecular Diels-Alder
reaction to afford highly substituted, enantiomerically enriched carbocycle 14. Further studies in both of these areas are planned
for the coming year, and we also intend to pursue applications of these
transformations to the synthesis of important natural products and other
targets of interest.
Copyright © American Chemical Society