AmericanChemicalSociety.com
Reports: AC4 48353-AC4: Light-Induced Ketonization - Elimination Reaction of Enol Forms of Gamma- and Delta- Hydroxy Ketones
Vladimir Popik, University of Georgia
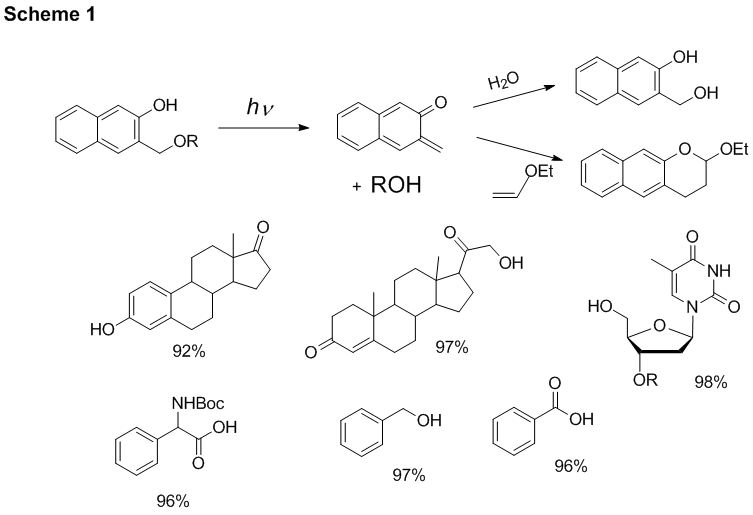
3-Hydroxy-2-naphthalenemethanol protecting group. We have shown that various alcohols and carboxylic acids can be protected with 3-hydroxy-2-naphthalenemethanol protecting group. The irradiation of such caged substrates results in an efficient release of the payload. The by-product of this reaction, o-naphthoquinone methide is hydrated to 3-hydroxy-2-naphthalenemethanol or can be trapped by ethyl vinyl ether (Scheme 1, yields of the photo-release reactions are shown under the substrates). We have also studied the rate of the release and have found out that substrates are released with time constant of 12 μs.
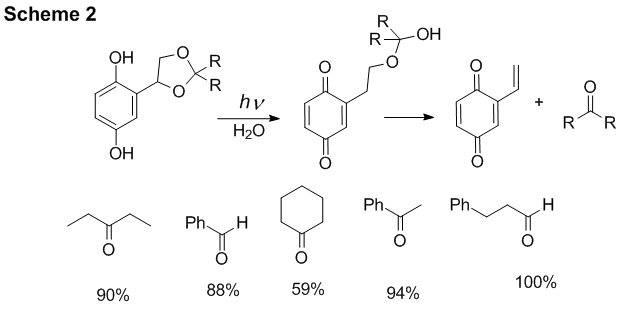
(2,5-Dihydroxyphenyl)ethylene glycol protecting group. We have previously shown that 2,5-dihydroxybenzyl protecting works well for caging of alcohols. Based on these results we have developed (2,5-dihydroxyphenyl)ethylene glycol protecting group for the photolabile protection of alcohols (Scheme 2).
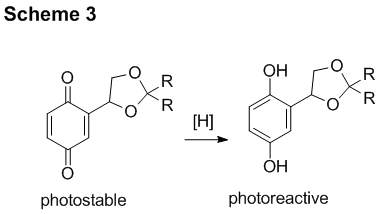
The additional advantage of this photolabile protecting group is the safety-catch option. An oxidized from of (2,5-dihydroxyphenyl)ethylene glycol acetals, the corresponding p-quinone, is photochemically stable but can be converted in to a photoreactive state by mild reducing agents (Scheme 3).
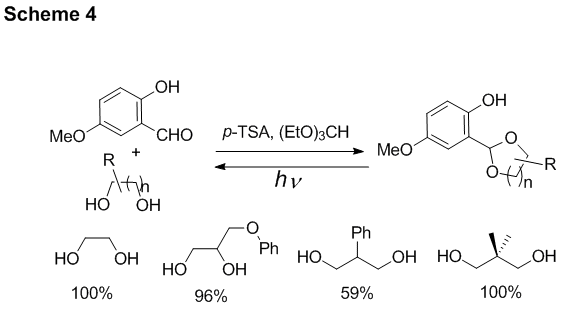
Protection of 1,2- and 1,3-glycols with salicylaldehyde derivatives. We have also developed the protecting group for 1,2- and 1,3-glycols, which is based on o-hydroxybenzyl alcohol reactivity. Thus, glycols readily react with 5-methoxysalicylaldehyde in the presence of dehydrating agents and catalytic amounts of p-toluenesulfonic acid to produce corresponding acetals (Scheme 4). 300 or 350 nm irradiation results in the efficient (F300 nm ~ 0.3) release of the substrate (Scheme 4).
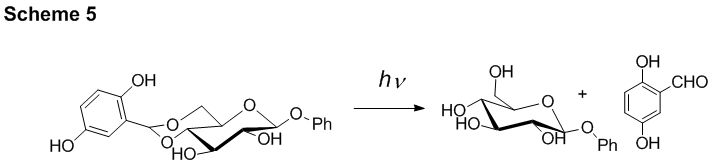
This methodology has been employed in the development of a safety-catch photlabile benzylidene protecting group for carbohydrates (Scheme 5).
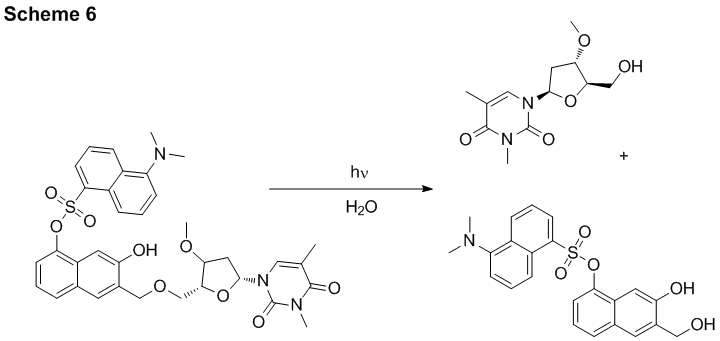
Photolabile protecting group with a fluorescent reporter. We have designed a photolabile protecting group that contains a dansyl fluorophore attached to it. Distribution of the compound caged with this PPG can be traced by the emission of the later. Dansyl group has been selected experimentally from many other fluorescent moieties as it does not interfere in the photo-uncaging step and is not bleached on the photorelease. For example thymidine derivative was efficiently (F350 nm ~ 0.28) released in 96% yield upon 350 nm irradiation of the caged compound (Scheme 6). Irradiation of the caged thymidine at 400 nm allows for measuring the fluorescence of the dansyl group but does not result in the release of the substrate.
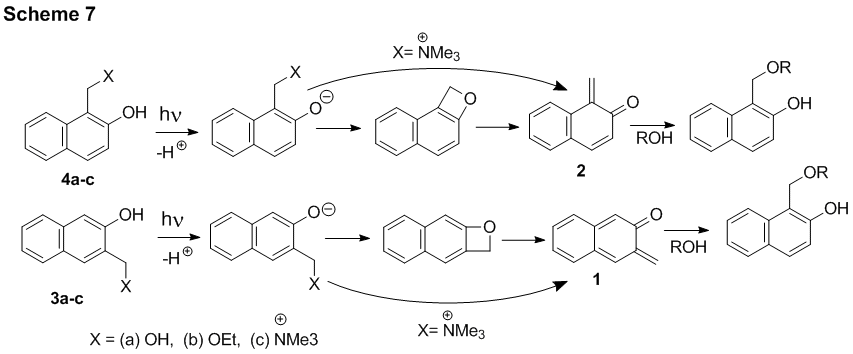
Mechanism of the dehydration of o-hydroxybenzyl alcohol derivatives. We have conducted detailed mechanistic studies of the formation and reaction of two isomeric naphthoquinone methides, 2,3-naphthoquinone-3-methide and 1,2-naphthoquinone-1-methide (Scheme 7). Laser flash photolysis of 3-hydroxy-2-naphthalenemethanol and 2-hydroxy-1-naphthalenemethanol allowed us to detect short-lived (t25oC ~ 12 ms) precursors of naphthoquinone methides. On the basis of the precursor reactivity and the results of DFT calculations, 2H-naphthoxete structure was assigned to these species (Scheme 7). In aqueous solution, naphthoquinone methides undergo rapid hydration to regenerate starting materials (tH2O = 5 - 7 ms at 250C). These reactive intermediates can be intercepted by other nucleophiles, such as the azide ion (kN3= 2.0 - 3.0 x 104 M-1s-1) or thiol (kSH = 2.2 -3.3 x 105 M-1s-1). It is interesting to note, that photolysis of ammonium precursors does not produce 2H-naphthoxete intermediate.
Copyright © American Chemical Society