AmericanChemicalSociety.com
Reports: UNI1 50126-UNI1: Redox-Switchable N-Heterocyclic Carbenes and their Corresponding Homo- and Heterometallic Complexes
Daniela Tapu, PhD, Kennesaw State University
In the first year of implementation time, effort and resources have been devoted to the project described in the original PRF proposal. Our project targets a new class of fused N-heterocyclic carbene ligands with a rigid bidentate architecture, ligands that we designed as supports for homo- and heterometallic complexes. The ligands incorporate redox active moieties. The reversible electrochemical oxidation and reduction of the redox moiety will provide a handle for additional fine-tuning of the carbene center and will offer the possibility to control the stoichiometric and catalytic reactivity of the corresponding transition metal complexes. Understanding the chemistry of these redox-active ligands will enable us to evaluate their potential role in applications, such as redox-switchable catalysts, that could allow for on-off switching of catalyst activity. In addition, this could provide a strategy for the separation of homogeneous catalysts from the products of a reaction. The chemical or electrochemical change in the oxidation state of the redox moiety would result in drastic changes in polarity and, as a result, the possibility to exert high control over the solubility of the catalyst. Ideally, the catalyst would be precipitated by electrodeposition and recovered for reuse, thus increasing cost-efficiency and reducing contamination of the reaction products with heavy-metal impurities. The underlying objective is to elucidate and learn to exploit the interrelationship between molecular architecture, electronic structure and chemical reactivity of these novel compounds.
The proposed synthesis of 2 by Buchwald-Hartwig amination did not lead to satisfactory results. However, the synthesis of 2 (R = neopentyl Np) was achieved by an alternate route as outlined in Scheme 1 using a sequence of reactions involving nitration, reduction of nitro groups, double acylation with pivaloyl chloride, reduction with LiAlH4 and cyclization with HC(OEt)3. The reaction of 2 (R = Np) with HBr was too slow. The reaction of 2 with BBr3 led to the desired salt 3. However, the purification and full characterization of 3 was impeded by low solubility in most organic solvents.
Compound 3 (R = Np) is very soluble in DMSO (but not too stable in DMSO, possible
due to oxidation) and sparingly soluble in acetone and acetonitrile.
The reaction of 3 with silver oxide
oxidized the redox-switchable moiety, but did not produce
the desired carbene silver complex (most likely due
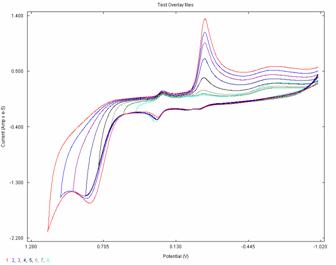
to steric hindrance). Preliminary cyclic voltammetry experiments were performed on 3 and showed that 3 has two electroactive sites (one
reversible DEp = 0.046 V, the other quasi-reversible DEp = 0.3795 V). Figure 1 shows the cyclic
voltammograms of 2 mM
solution of 3 at a glassy carbon electrode,
in buffered solutions (pH 3.5) with a decrease in the scan window from 1.250 to
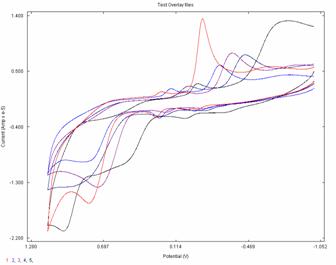
0.850 V. Cyclic voltammograms of 2 mM solution of 3 at various pHs (same ionic strength) (pH =
2.5, 3.5, 4.8, 5.5, 8.0) are shown in Figure 2. The reduction potentials
are shifting to more negative values with increase pH. Currently we are working
on the synthesis of other salts of type 3
with different substituents on the two nitrogen atoms
in order to improve solubility and to decrease steric
crowding around the carbene center. Fig.1. Fig.
2 This PRF grant allowed five undergraduate students to participate in
research, both as independent studies for credit during Fall
and Spring semester and as paid stipends over summer. Two of them have
graduated in spring 2010. Uyen Do was accepted in
dental school and Gerhard Kummerow is currently
preparing his application for graduate school in chemistry. Chris Ghattas, Mahatab Chowdhury and Gary Richoux will continue to work on this project for another
year. Due to his academic achievements and his involvement in undergraduate
research, Chris was awarded one of the National Science Foundation scholarships
available in our college and was the recipient of the Merck award for
achievements in organic chemistry in Spring 2010. The
research infrastructure in my lab received a boost by the acquisition of a new
dry-box. This dry-box is mainly used for carbenes' chemistry. All five students
were trained to use it. After an initial
training period, the students were actively involved in the preparation and
characterization of the desired compounds. We plan on presenting the initial
results of our work at the Southeast Regional Meeting of the American Chemical Society
in

Copyright © American Chemical Society

