AmericanChemicalSociety.com
Reports: GB1 48347-GB1: New Synthetic Methodology for Ring Formation
Elizabeth A. Colby Davie, PhD, Assumption College
Statement of Progress and Significance
This year, research has been conducted on both projects described in the original PRF proposal (the synthesis of schischkiniin and the synthesis of cyclobutanones and lactones via nucleophilic catalysis) as well as a third project that was inspired by work on schischkiniin and reported in the last progress report (bidirectional synthesis of montamine derivatives). This PRF grant enabled three students to conduct research as independent studies for credit during the 2009-2010 academic year. Of these students, two were accepted to graduate programs in chemistry and began their studies in the fall of 2010. Importantly, the PRF grant also provided funding for two students for a 10-week research period over the summer. They joined two other students funded by Assumption College to form an effective summer research group. During this time, a great deal of data was collected. While not all of our experiments were successful, the students learned a great deal from their experiences and have moved the projects forward.
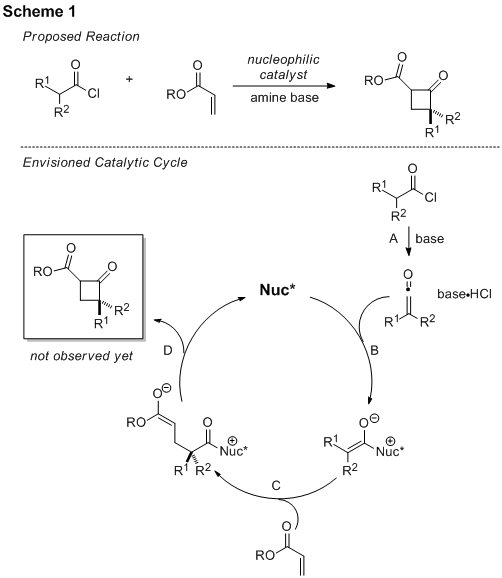
I. Enantioselective Nucleophilic Catalysis – Synthesis of Cyclobutanones and γ-Lactones
During the summer of 2009, a detailed investigation of the proposed nucleophile-catalyzed reaction to produce cyclobutanones (Scheme 1) was undertaken. The student who was involved with this work continued her research during the spring semester of 2010 as part of a college honors project. Initial work was done that focused on repeating similar chemistry in our laboratory: the formation of a β-lactone from an acid chloride and an aldehyde. Using what we found from that early work, we performed many experiments designed to carry out the process from Scheme 1. Propionyl chloride was identified as a good ketene precursor, and a variety of α,β-unsaturated ketones and esters were investigated as coupling partners. Solvent, temperature, and Lewis acids were also screened. To our disappointment, only oligomers of propionyl chloride have been isolated and no cyclobutane products have been observed, leaving the desired process elusive so far.
II.
Synthesis of Schischkiniin Work
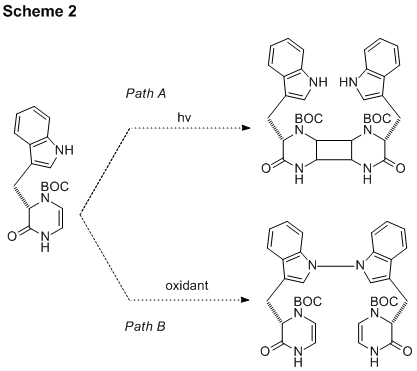
on the synthesis of schischkiniin this year began with an investigation of the
oxidative dimerization process to install the N-N bond (Scheme 2, path B). A simple model indole system was selected for
these studies. A variety of oxidizing
reagents were employed, but most conditions led to a complex mixture of
products. In the future, we plan to
investigate electrochemical methods for this transformation. During
the previous summer, a key intermediate was synthesized for the [2+2]
cycloaddtion in the planned synthesis of schischkiniin. This progress allowed further investigation
of our strategy during the past year. We
aimed to remove the protecting group and carry out photocycloadditions on the
free dihydropyrazinone (Scheme 2, path A).
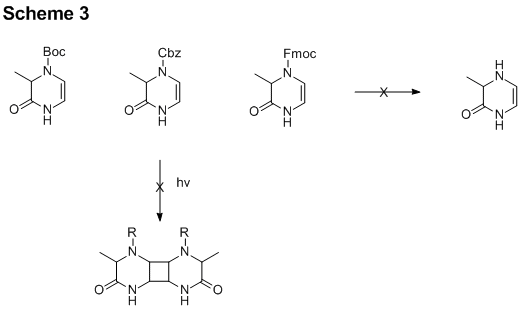
To do so, several analogs were prepared with different protecting groups
to produce three protected dihydropyrazinones (Scheme 3). Each of these compounds was subjected to
appropriate reagents for the removal of the protecting group, but in no case was
the desired deprotected analog isolated.
One problem is that it appears that this compound and/or byproducts are
water soluble. Another issue is likely decomposition
of the deprotected compound. Given these
problems, we chose to focus on the protected variants to further probe the
cycloaddition chemistry. These efforts
were met with little success, despite utilizing several different wavelengths
of light, catalysts, solvents, and reaction times. At
this point, our synthetic route seems difficult. However, we plan to revisit one more tactic
for the deprotection of the Boc-protected dihydropyrazinone – the use of a
solid-supported acid (acidic amberlyst resin).
The free dihydropyrazinone should have less steric hindrance and
participate in a cycloaddition more readily than the protected variants. III. Synthesis of Montamine Derivatives Using a
Bidirectional Synthetic Strategy As
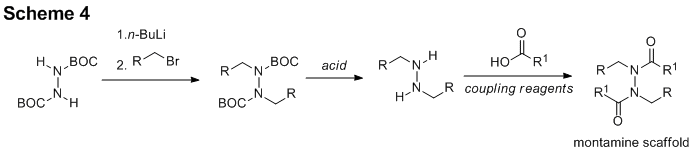
reported in the last update, the schischkiniin project spawned a new project idea,
the bidirectional synthesis of montamine and derivatives (Scheme 4). The most success has been realized on this
new project. Each step of the envisioned
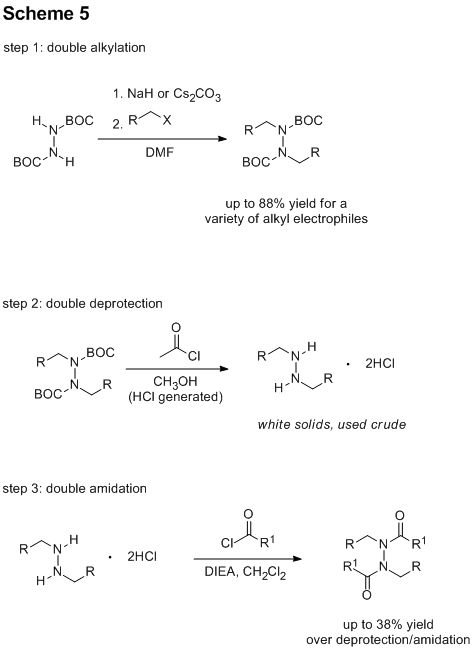
three-step process has come to fruition with model substrates (Scheme 5). Notably, we have achieved excellent yields
with alkyl iodides in a double alkylation process. Reliable and convenient removal of the Boc
protecting group from these products has been achieved by treatment with acetyl
chloride in methanol producing the corresponding dihydrochloride salts. We have found that the crude dihydrochloride
salts can be used directly in amidation reactions. While reasonable yields for a double reaction
have been achieved in this amidation process (30-38% yield), more work on the
project remains at optimizing the reaction.
To date, we have synthesized a compound that brings in much of the general
aromatic portions of montamine. In the
future, we plan to extend the strategy to the natural product, montamine. Summary and a Final Word
Regarding Significance Much
has been investigated with respect to the original two projects that were
proposed. Both projects have hit major
challenges, but have still allowed students to experience firsthand how
research is conducted, including the highs and lows that every scientist encounters. By working on these projects, many literature
searches have been conducted that have directly led to the identification of a
third, related project (synthesis of montamine) that has been very fruitful and
educational for the students. The
flexibility of the PRF grant has been crucial for establishing a thriving
undergraduate research program at Assumption College – both the students and
principal investigator are deeply grateful for this support.
Copyright © American Chemical Society