AmericanChemicalSociety.com
Reports: DNI1 49181-DNI1: New Catalytic Asymmetric Reactions of Allylsilanes and Isatins
Annaliese K. Franz, PhD, University of California (Davis)
Enantioselective catalysis is the most efficient method to construct the carbon-carbon bonds of stereochemically-defined molecules from simple feedstock chemicals. Challenges remain to design and develop efficient catalysts with low catalyst loading, provide synthetic methods that acheive high enantioselectivity, incorporate polar groups and heterocycles, and determine the effects of additives and counterions. The goal of this research is to develop new catalytic asymmetric reactions of allylsilanes and isatins using the reactivity of chiral Lewis acid complexes with chelating electrophiles for the efficient synthesis of complex heterocycles from simple feedstock chemicals.
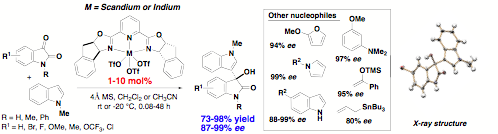
In this granting period, we
have compared the activity and selectivity of diverse Lewis acid catalysts and
shown that chiral scandium(III)- and indium(III)-pybox complexes as efficient
catalysts for the direct enantioselective addition of a broad scope of indoles
and electron-rich pi-nucleophiles to isatin electrophiles. The reaction proceeds
under mild conditions using a Sc(III)-pybox complex with as low as 1 mol%
catalyst loading. While catalytic asymmetric additions to isatins have been
reported previously, this direct method represents the first catalytic
asymmetric addition of indole nucleophiles to an isatin. This operationally-simple method does
not require the use of activated arenes or transmetalation conditions. High
yields and excellent enantioselectivity are achieved for various substitution
patterns, including both NH isatins and N-alkylated isatins, which are directly applicable for
the synthesis of natural products and biologically active oxindoles. Less
reactive isatin electrophiles required up to 10 mol% catalyst loading, but
continue to show high enantioselectivity even at room temperature. The same scandium(III)-pybox complex is
also successful for allylation and aldol reactions.
The structure and absolute configuration were confirmed by x-ray analysis. Mechanistic insight was gained using electrospray ionization mass spectrometry (ESI-MS) for the analysis of dynamic intermediates. Mixtures of 5-bromo-N-methylisatin, Sc(OTf)3 and inda-pybox ligand were injected into the MS to observe binding of the scandium complex to the isatin electrophile. Peaks at m/z = 736.5, 799.8, and 877.5 were detected, which correspond to [Sc(OTf)2pybox]+, [Sc(OTf)2(CH3CN)+Na]+, and [Sc(OTf)(CH3CN)pybox(isatin)-CH3+Na]+ complexes.
As part of this investigation, the reactivity and selectivity of Sc(III)-inda-pybox and In(III)-inda-pybox complexes were compared. For indoles and other aromatic nucleophiles, indium(III) complexes also catalyzed the Friedel-Crafts reaction with comparable results. While both catalysts are suitable for Friedel-Crafts reactions of aromatic nucleophiles, it was remarkable that Sc(III)-inda-pybox was also successful for aldol and allylation reactions of silylenolethers and allylstannanes. Because 1,2-dicarbonyl compounds are important electrophiles, this reactivity and selectivity comparison for various Lewis acid complexes will help guide selection of appropriate Lewis acid in reactions of other 1,2-dicarbonyls. The choice of chiral metal complex proved to be important for yield and enantioselectivity, where the inda-pybox ligand provides high enantioselectivity with several metal complexes. Further optimizations to the catalyst have extended this methodology for efficient reactions of allylsilanes and other nucleophiles, for which the scope is currently being investigated.
We have extended the Lewis acid activation of isatin electrophiles to develop a highly regio- and stereoselective cyclization of 5-alkoxy-2-aryloxazoles to provide a new class of spirooxindole-oxazolines. Titanium-catalyzed conditions provide excellent yields and up to >99:1 diastereoselectivity. The reaction is remarkable that the simple switch from an H or methyl substituent to an isopropyl substituent at the 4-position of the oxazole provides a regiochemical reversal and exclusive access to either the 2- or 4-functionalized spirooxazolines. Preliminary investigations with chiral Lewis acid catalysts have resulted in enantioselectivity of 70% ee.
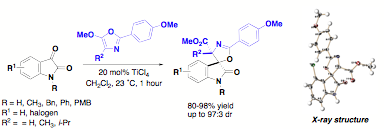
These synthetic and structural
studies contribute to a general understanding of activation and selectivity for
chiral Lewis acid complexes with chelating electrophiles. This funding has also
contributed to the training of six students, including five graduate students
and one undergraduate student. Of
these students, one student applied for and received an NSF graduate research
fellowship. Two students have
presented their research at ACS National meetings in the form of one oral
presentation and two poster presentations. One publication has resulted from
this work. One additional publication is submitted and two additional
manuscripts are in preparation.
Copyright © American Chemical Society

