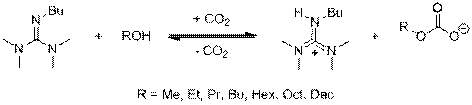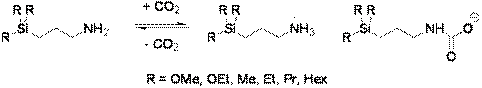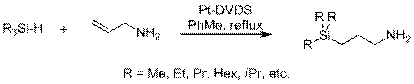AmericanChemicalSociety.com
Reports: ND10 49126-ND10: Sustainable Synthesis of Nanoparticles
Charles Liotta, PhD, Georgia Institute of Technology and Charles A. Eckert, PhD, Georgia Institute of Technology
The primary goal of this project is to combine reaction and separation to develop a sustainable synthesis of monodisperse nanoparticles using a recyclable solvent system. Our research group has pioneered a class of switchable solvents known as reversible ionic liquids (RevILs), shown in Figure 1.1
Figure 1. RevILs formed from guanidine 2-butyl-1,1,3,3-tetramethylguanidine (TMBG).
When CO2 is bubbled through an equimolar mixture of a straight-chain alcohol and a guanidine, the neutral molecular liquids are converted into an ionic liquid, possessing greatly different properties from those of the molecular precursors. Heating or sparging with an inert gas drives off the CO2 and regenerates the molecular precursors—this process can be repeated numerous times.2 We have also developed 1-component RevILs—trialkylsilylpropylamines, which react with carbon dioxide to form a carbamate anion and an ammonium cation, as in Figure 2.3
Figure 2. One-component RevILs.
Using the one-component RevILs eliminates the need for precise equimolar amounts of molecular precursors. The trialkylsilylpropylamines are not commercially available, but are easily synthesized in house via a platinum-catalyzed hydrosilylation reaction, seen in Figure 3.
Figure 3. Synthesis of trialkylsilylpropylamines. Reaction time varies based on silane substituents.
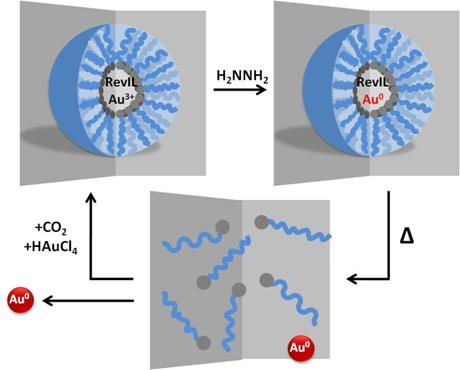
Our system uses reverse micelles as templates to control the size of the nanoparticles formed, shown in Figure 4.
Figure 4. Reverse micelle containing gold salt and RevIL; reduction of gold salt to form gold nanoparticles; deposition of nanoparticles; recycle.
Our goal is a fully recyclable system, requiring only the addition of more gold salt and CO2 to reform the reverse micelles and prepare the system for another cycle.
<>Results and Discussion
After successfully showing the formation, dissolution, and reformation of the reverse micelle system with methyl orange, we investigated the formation of gold nanoparticles. We varied the amounts of the system components (see Table 1) and found that we were able to recycle one system (Vial 2) twice.
Table 1. Testing parameters of [TMBGH+][MeOCOO-]/HAuCl4/N-propyl-octylsulfonamide system in 15 mL dodecane.
Vial | Surfactant (g) | RevIL (g) | HAuCl4 (g) | WHAuCl4/WRevIL |
1 | 0.133 | 0.040 | 0.0019 | 0.048 |
2 | 0.066 | 0.022 | 0.0027 | 0.123 |
3 | 0.030 | 0.041 | 0.0024 | 0.058 |
4 | 0.131 | 0.051 | 0.0020 | 0.039 |
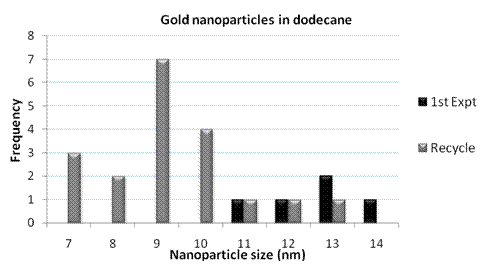
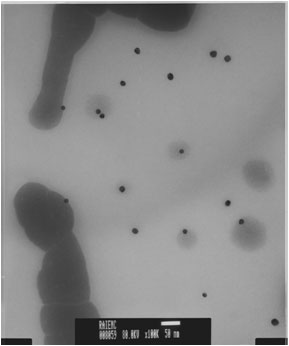
The nanoparticles were then imaged via TEM and found to have a size range of 7 to 14 nm (analyzed via ImageJ).
Graph 1. Range of nanoparticle sizes observed via TEM for initial experiments in dodecane.
The recycles showed a similar size range; however, it is important to note that our sample sets were very small—i.e., the solution of nanoparticles was highly dilute.
As the surfactant was more soluble in hexane, this was used as the continuous phase in subsequent experiments, allowing us to reach higher concentrations of nanoparticles both in solution and on the TEM grids.
Figure 5. Gold nanoparticles synthesized in second round of experiments. Note dark areas, presumed to be organic matter.
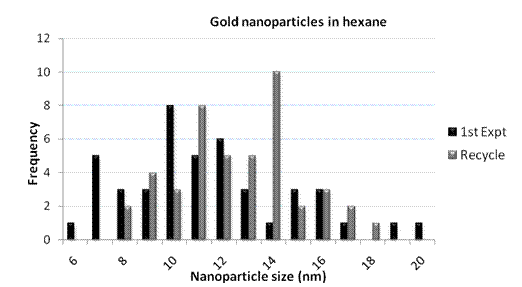
Imaging revealed that more nanoparticles were formed, with a size range of 6 to 20 nm, as seen in Graph 2. Promisingly, the size ranges for the initial experiment and the recycle are similar.
Graph 2. Range of nanoparticle sizes observed via TEM for experiments in hexane.
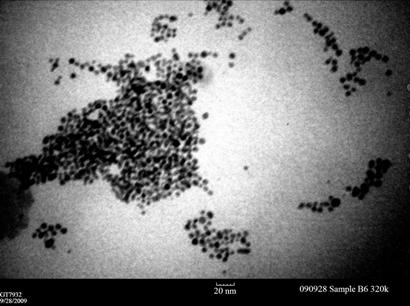
We have begun capping the nanoparticles with dodecanethiol after reduction, but prior to deposition; although this renders the system unsuitable for recycle, it does allow for better imaging, as in Figure 6.
Figure 6. Dodecanethiol-capped gold nanoparticles. Stabilization allows for better imaging.
We have also carried out preliminary experiments using 1-component RevILs and gold (III) acetate [Au(OAc)3], and have been able to successfully synthesize nanoparticles using this system. Additionally, we have found that using RevILs with long alkyl chains on the silicon—for example, trihexylsilylpropylamine—eliminates the need for a surfactant. Changing the alkyl substituents of the RevIL could also allow us to change the size and shape of the reverse micelle formed.
In conclusion, (1) we have successfully formed gold nanoparticles using the two component reversible ionic liquid and (2) we reversed, reformed and recycled our system multiple times—demonstrating the formation of nanoparticles.
<>Path Forward
Our immediate focus for this project is successful deposition and imaging of the nanoparticles synthesized. We will then turn our attention to the numerous variables at work in our system. The initial factors under consideration include:
· Weight ratio of gold salt to RevIL
· Concentration of RevIL and gold salt in continuous phase
· Type and amount of reducing agent (e.g. hydrazine in THF, H2)
Each of these factors will be systematically changed until we can consistently form monodisperse nanoparticles over several cycles.
We plan to use the optimized reaction conditions from the gold nanoparticle synthesis to synthesize platinum and palladium nanoparticles; we will then use these nanoparticles in proof of concept reactions to test their activity. For palladium, we plan to look at the catalytic hydrogenation of 4-methoxyacetophenone; for platinum, we plan to use the catalytic hydrogenation of nitrobenzene to aniline.
<>Works cited
1. (a) Jessop, P. G.; Heldebrant, D. J.; Li, X. W.; Eckert, C. A.; Liotta, C. L., Green chemistry - Reversible nonpolar-to-polar solvent. Nature 2005, 436 (7054), 1102-1102; (b) Phan, L.; Chiu, D.; Heldebrant, D. J.; Huttenhower, H.; John, E.; Li, X. W.; Pollet, P.; Wang, R. Y.; Eckert, C. A.; Liotta, C. L.; Jessop, P. G., Switchable solvents consisting of amidine/alcohol or guanidine/alcohol mixtures. Industrial & Engineering Chemistry Research 2008, 47 (3), 539-545.
2. Hart, R.; Pollet, P.; Hahne, D. J.; John, E.; Llopis-Mestre, V.; Blasucci, V.; Huttenhower, H.; Leitner, W.; Eckert, C. A.; Liotta, C. L., Benign coupling of reactions and separations with reversible ionic liquids. Tetrahedron 2010, 66 (5), 1082-1090.
3. Blasucci, V.; Dilek, C.; Huttenhower, H.; John, E.; Llopis-Mestre, V.; Pollet, P.; Eckert, C. A.; Liotta, C. L., One-component, switchable ionic liquids derived from siloxylated amines. Chemical Communications 2009, (1), 116-118.
"Acknowledgment is made to the Donors of the American Chemical Society Petroleum Research Fund for support (or partial support) of this research."
Copyright © American Chemical Society