AmericanChemicalSociety.com
Reports: B1 46293-B1: Development of Synthetic Tools Using Ynol Ether Ionic Chemistry
Benoit Daoust, Universite du Quebec a Trois-Rivieres
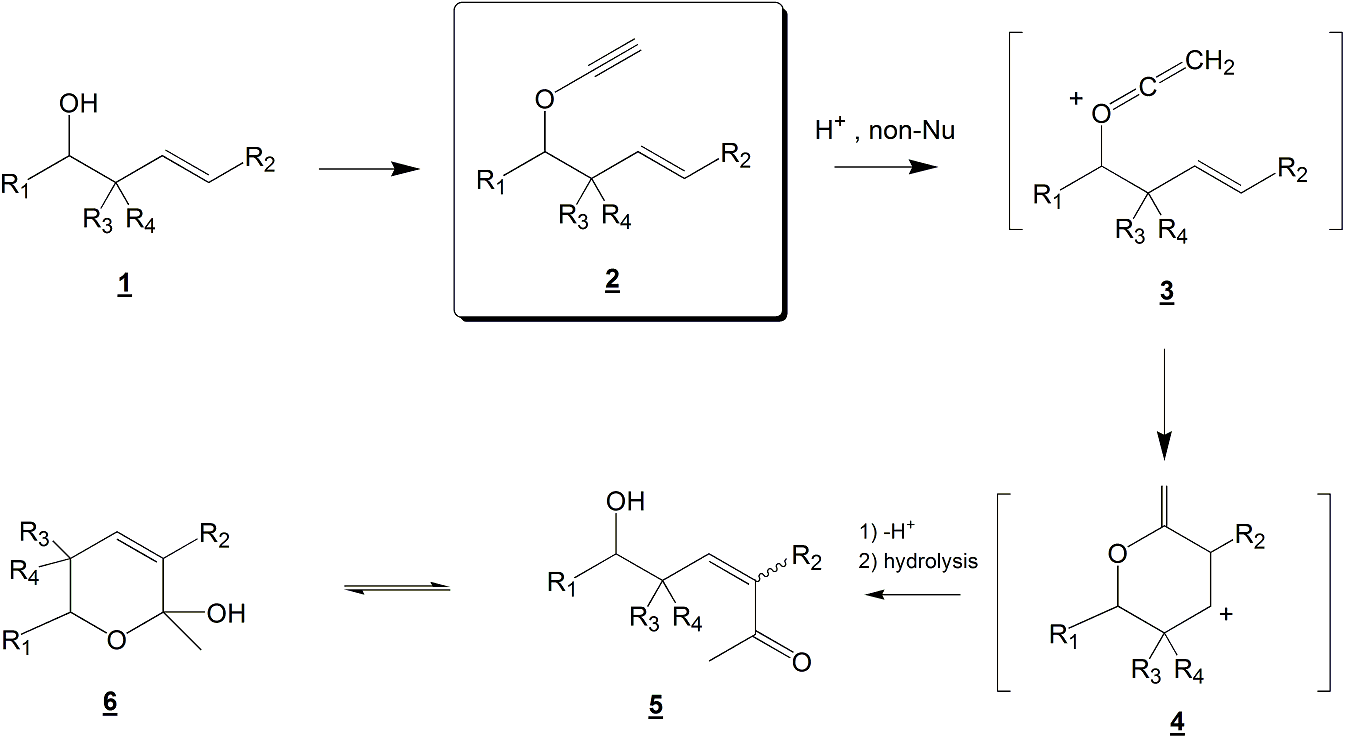
Our objective is to study the intramolecular reaction of homoallylic ynol ethers 2 (see Scheme 1) with electrophiles in non-nucleophilic media. The purpose is to understand the reactivity of ynol ether derived cationic ketene intermediates 3 with strategically placed internal nucleophiles. This strategy should allow us to form, via the carbocation 4, unsaturated ketones 5 and/or hemiketal 6.
Scheme 1
In the first two years of this project, we prepared models and tried to make them react in order to produce the desired compounds 5 or 6. Unfortunately, after a lot of efforts (see annual reports of 2008 and 2009), we were not able to form the desired molecules. During the first two years, we only tested molecules having an alkyl group at position R2. In this third year, we decided to put an alkoxy group at position R2, thus enhancing the nucleophilic character of the double bond. This, we hope, might help achieving our goal.
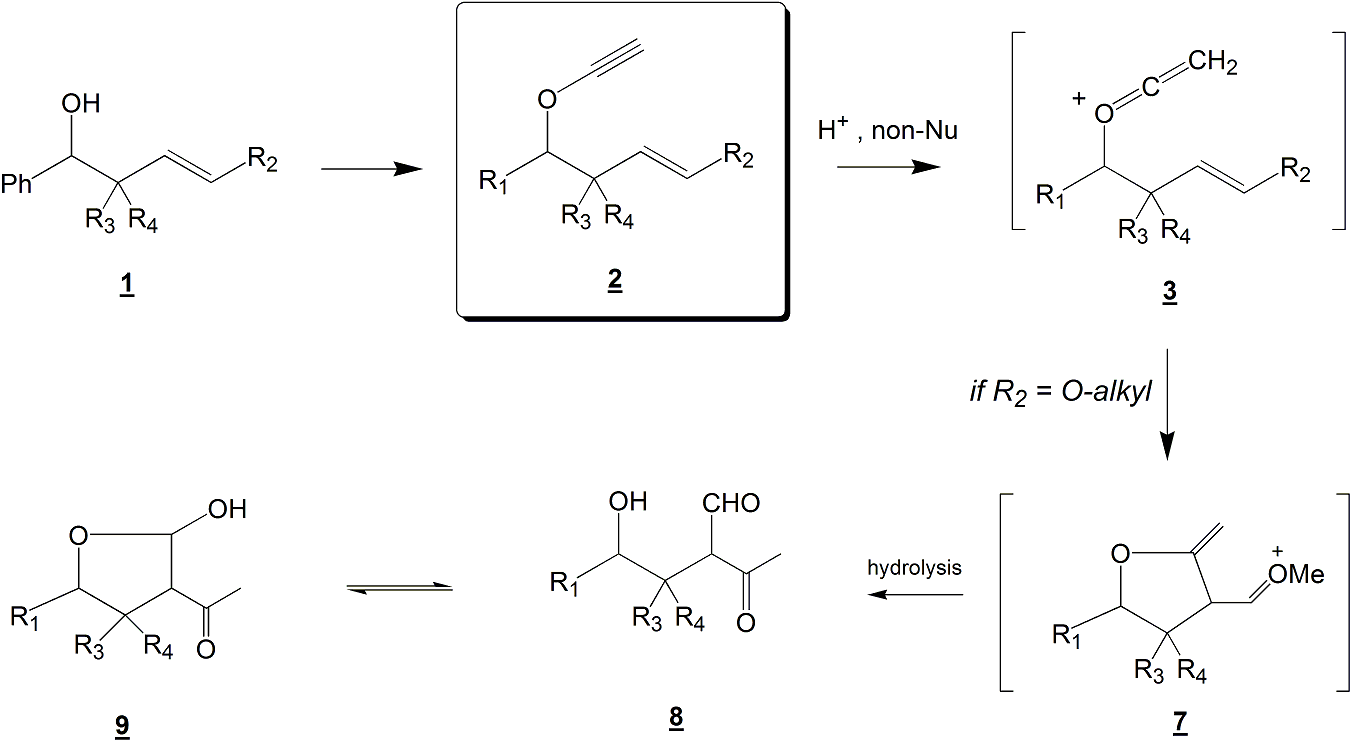
However, the presence of this alkoxy group (now an enol ether moiety instead of a simple alkene) will force the reaction to occur via a 5-membered ring carbocation intermediate 7 (see Scheme 2), in order for the positive charge to be delocalized. The desired products in this specific case will be molecules 8 or 9.
Scheme 2
Simon Ricard and Jodrey Bergeron, two new students in this project, first synthesized the desired models, 1a (R1=Ph, R2=OMe, R3=H, R4=Me) and 1a (R1=Ph, R2=OMe, R3=R4=Me) using known methods (ref. 1). Characterizations of molecules 1a and 1b were quite difficult due to their instability towards traces of acid. However, Simon and Jodrey finally got the twist with these compounds by avoiding any contact with possible sources of acidity (use of base-treated silica, elimination of any CHCl3 or CDCl3, ...). They were just able to finish the preparation of these two models before they left the lab.
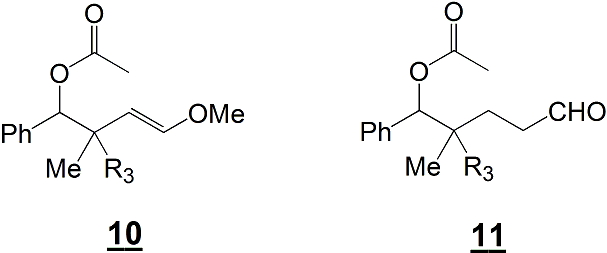
Simon Lessard took over and was able to study the cyclization of these models. In order to produce the desired compounds, he used different non-nucleophilic medium acidic medium : Montmorillonite H+ resin, TsOH, camphorsulfonic acid, HgO, Hg(OAc)2, Ru(p-cymene)Cl2 (ref. 2 and 3) and more. Unfortunately, in all these cases, no desired products were observed. We could only detect compounds resulting from the hydrolysis of the enol ether moiety and/or of the ynol ether moiety (this hydrolysis is most probably due to the aqueous work-up) (see Scheme 3, compounds 10 and 11). We were able to observe that the ynol ether moiety was hydrolyzed more readily than the enol ether counterpart.
Scheme 3
Since cyclization did not occur, we were not able to publish any of these results. I was able however to present some of the above results within a communication concerning a different project in Toronto in 2010 (ref. 4).
References
(1) (a) Gung, B.W.; Smith, D.T.; Wolf, M.A. Tetrahedron 1992, 48(26), 5455-5466 (b) Kim, S.; Kim, Y.C. Tetrahedron Lett. 1990, 31(20), 2901-4 (c) Quintard, J.-P.; Dumartin, G.; Elissondo, B.; Rahm, A.; Pereyre, M. Tetrahedron 1989, 45(4), 1017-28.
(2) Kita, Y.; Maeda, H.; Omori, K.; Okuno, T.; Tamura, Y. Synlett 1993, 273-4.
(3) Kita, Y.; Maeda, H.; Omori, K.; Okuno, T.; Tamura, Y. J. Chem. Soc. Perkin Trans. 1 1993, 2999-3005.
(4) Rahem, N.; Daoust, B. Synthesis of Amino Acid Precursors and γ,d-Unsaturated Carbonyls via a Copper Coupling - Claisen Rearrangement Sequence, 93rd CSC Conference & Exhibition, Toronto (Ontario), June 2010.
Copyright © American Chemical Society