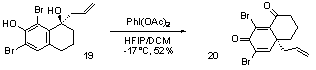AmericanChemicalSociety.com
Reports: B1 48122-B1: Cycloaddition Reactions for the Formation of Indolines, Indoles and Benzofurans, and Subsequent Functionalization Reactions
Sylvain Canesi, Universite du Quebec a Montreal
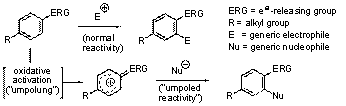
This research report focuses on the accomplishments achieved during the second year of this grant: The central theme of our research is what may be termed "aromatic ring umpolung." Whereas an electron-rich aromatic nucleus normally reacts as a nucleophile, suitable oxidative activation can convert it to a reactive electrophilic intermediate, which may be intercepted with external nucleophiles, Scheme 1. Our research focuses on the capture of such intermediates with carbon nucleophiles, leading to the formation of new C-C bonds. Among the various reagents that could be used in the oxidative step,hypervalent iodine complexes were found to be the agents of choice. Scheme 1. This method requires no metal catalysts, it does not need activated substrates, and it is environmentally benign. This inversion of polarity generates a phenoxonium ion 2 allowing to transpose cationic transformation in aliphatic chemistry to the aromatic chemistry via an oxidative process. Such an approach has been investigated to perform an oxidative pinacol process via a 1-2 group shift. One of the most remarkable transformations in synthesis is probably transposition. This reaction allows the transformation of a simple structure into a variety of more complex motifs. The Wagner-Meerwein transposition is among the most well known of these rearrangements and an oxidative extension has been demonstrated. Preliminary results have already shown significant results that could lead to a plethora of highly functionalised systems present in several natural products. Indeed, the 1-2 migration of aryl, allyl and alkyl groups has been observed on an array of different phenols, Table 1. As expected, during the oxidation of compound 1h the migration of the n-butyl group is very predominant; only a small amount of methyl migration is observed (~5%). This oxidative process seems to occur as a regular rearrangement applicable to a large scope of aryl, allyl and alkyl groups. Indeed, the same rules as the ones applying to a conventional Wagner-Meerwein transposition are observed. It should be stressed that this process produces, in one step, highly functionalised cores. Indeed, compounds such as 3d, 3e and 3f have a quaternary carbon center connected to four sp2 carbons, Table 1. In addition, this transposition has been extended to bicyclic phenols such as tetralone 4. With good migrating groups such allyl and aryl, the formation of dienone 5 is observed but in the case 4c (R=Me), a ring contraction occurs to produce 6. These results, leading rapidly to an interesting bicyclic core, demonstrate the potential of this transformation and its potential application in synthesis, Table 2. In addtion, an interesting transformation is observed by oxidation of the protected alcohol 7 leading to the acetal 10. Indeed, the allyl C-C bond fragmentation would lead to the species 9 that would be trapped by the acetic acid released during the reaction. This transformation is similar to an oxidative pinacol/acetalisation tandem process, Scheme 2. In presence of a substituent such as allyl or propargyl groups, due to a potential concerted mechanism.This rearrangement process can be extended to an unprecedented 1,3-allyl shift via a chair transition state. The transposition/acetalisation process described previously has also been extended, Scheme 3. Oxidation of compound 14 produces the desired allenic moiety with a noteworthy global yield of 72%. This process seems to occur more efficiently with a propargylic group than an allylic group. This aspect could be rationalized by the linear geometry of the sp carbon center (half chair transition state) that would be more oriented to interact with the phenoxenium ion generated. We have also been interested in trapping the corresponding oxonium generated on the side chain with an intramolecular nucleophile. The oxidation of 17 leads to the acetal 18 via a cascade process. Moreover, this transposition can be accomplished on a bicyclic phenol 19; the introduction of bromines in ortho position is required to force the allylic group to react in para position. We suppose that the migration of the allylic group should occur stereospecifically with a retention of configuration, due to the concertness of the mechanism involved, Scheme 4. In summary, an oxidative transposition process allows a rapid access to highly functionalized synthons containing a dienone, a quaternary carbon center directly connected to several sp2 carbons. This transformation provides new strategic opportunities in the chemical synthesis of natural products. It should be noted that the undergraduate students that have been involved in this project have pursued their studies in master degree.
Copyright © American Chemical Society