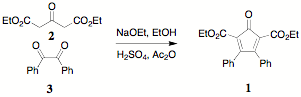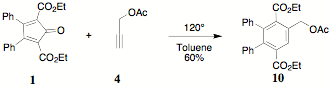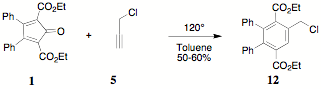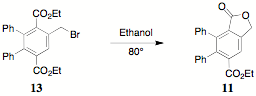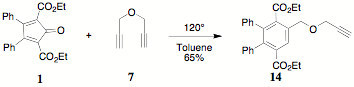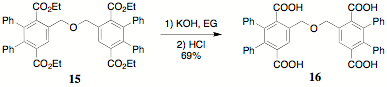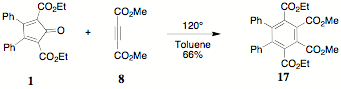46413-B1
The Synthesis of Novel Indenofluorenes
Carboxylated
cyclopentadienones (CCPD) react readily in Diels Alder reactions with inverse
electron demand (dienophile is electron rich). They present many intriguing
possibilities. The functionalization provided by the dienophile (propargyl
derivatives) in Diels-Alder reactions with the carboxylated cyclopentadienone
(CCPD), The
synthesis of 1 follows an Aldol
condensation pathway that employs diethyl (or dimethyl) acetonedicarboxylate 2 and benzil 3. The unsubstituted CCPD 1
(X = H) has been dubbed "Orange" because of its color and has generally been
the basis for preliminary research and continues to be a versatile starting
material for many applications. The synthesis of 1 has recently been carried out on 50 g scale and its
purification (recrystallization vs chromatography) has been improved.
The
current reactions of CCPDs are centered on the Diels Alder reaction with the
propargyl systems propargyl acetate 4,
propargyl chloride 5, propargyl
bromide 6, propargyl ether 7, dimethyl acetylenedicarboxylate 8 and trispropargyl amine 9.
The
reaction of 1 and propargyl acetate 4 provided diethyl
2,3-diphenyl-5-acetoxymethylterephthalate 10. The acetate could be characterized by 1H
NMR absorptions and integrations for the acetate methyl (2.13 d), the benzylic methylene (5.27 d), the ethyl ester methyl (0.89 d) and methylene (3.98 d) and appropriate aromatic absorptions,
especially the singlet (7.86 d).
The
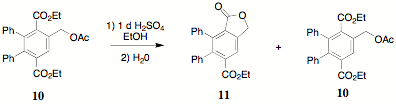
acetate 10 was intramolecularly
transesterified with sulfuric acid to generate a 50/50 (by 1H NMR)
mixture the lactone 11 and the
starting material 10.
Characterization of 11 was based
on the lactone singlet (5.38 d).
The
reaction of propargyl chloride 5 and 1 produced diethyl
2,3-diphenyl-5-chloromethylterephthalate 12. Characterization of 12 followed that associated with 11. The 1H NMR absorption and relative
integration of the benzylic methylene (4.77 d) of the chloromethyl substituent clearly distinguishes 11.
The
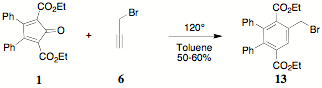
reaction of propargyl bromide 6 with 1 cleanly provided the bromomethyl derivative,
diethyl 2,3-diphenyl-5-chloromethylterephthalate 13. The characterization of 13 was not, however, straightforward. The crude
product exhibited the expected 1H NMR absorptions and integrations
but after recrystallization from ethanol, a 75% recovered yield of 11 was obtained. Furthur investigation of the lactone
formation is underway.
The
use of propargyl ether 7 as a
Diels-Alder reactant with 1 was
investigated from two positions. Initially, a calculation error in the ratio of
7 to 1 (10:1 was used instead of 1:1) produced the
monoreacted propargyl substituted terephthalate 14. The characterization of 14 by 1H NMR was based on the appearance of
an absorption for the benzylic methylene (3.99 d) and the propargylic methylene (4.06 d) as well as the terminalm alkyne proton (2.19 d) in addition to the related terephthalate
absorptions..
This
error had the unintended consequence of providing an entry into a series of
compounds containing pendent ethynyl units, a process usually associated with
protection-deprotection schemes. It appears that this can be a preparative
method as long as the boiling point of the diyne is not excessive.
The
reaction of 7 and 1 in a 1:2 ratio provided the bisterephthalate 15. The usual characterization was used to establish
the absence of a pendent propargyl unit in the structure.
A
preliminary probe of the suitability of the polyterephthalates for conversion
to the corresponding indenofluorenones was conducted using 15. The hydrolysis of 15 was carried out to yield the tetraacid 16. It could be established that the benzylic ether
bridge between the terephthalate moieties was intact. Furthur studies on this
system (intramolecular ring closure) are underway.
The
reaction of dimethyl acetylenedicarboxylate 8 with 1 was undertaken
to provide access to a polysubstituted product with wide potential. The
characterization of 17 was
easily established by 1H NMR. Appropriate ethyl ester and methyl
ester absorptions and integrations were observed.
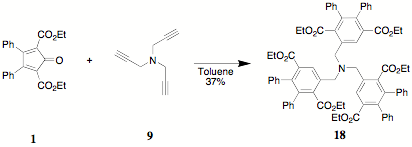
An
entry into the polysubstituted terephthalate systems was established by the
reaction of 9 with 1. The reaction was staged (80°/24h, 120°/24h) to
prevent the loss of the volatile 9.
Future reactions involving 9
will be conducted in closed systems. The characterization of the congested
triterephthalate 18 was based on
surprisingly clear 1H NMR absorptions and integrations for the
benzylic amine methylenes (3.85 d),
the ester methylenes (4.01 d), the
ester methyls (0.88 d), and in
particular the singlet associated with the lone proton on the terephthalate
ring (8.11 d).
The
reactions outlined in this report set the stage for the investigation of 1)
additional CCPD substitution by way of substituted benzils, 2) the use of
polypropargyl substrates in the preparation of polyterephthalates and 3) the
manipulation of polyterephthalic acids as precursors to polyindenofluorenones.