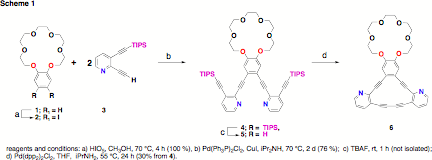46543-B4
Ion Sensitive Push-Pull Pyridoannulenes
The goal of this work is for undergraduates to synthesize a series of annulenes and test their spectroscopic properties in the presence of various metal cations. Towards this goal, annulene 6 was successfully produced. The synthesis is shown in Scheme 1.
First, commercially available
benzo-18-crown-6 was treated with periodic acid in methanol. The result of this reaction was the
facile isolation of diiodide 2 in nearly quantitative yield and in sufficient
purity that no contaminants could be detected by either 1H or 13C
NMR spectroscopy. This compound
underwent Sonogashira coupling with two equivalents of 3 to yield 4 in 71 % yield. Compound 3 was prepared from commercially
available 2-bromo-3-pyridinol in three steps using a procedure previously
published from our lab. Several
attempts were made at this reaction. In the first attempts at this reaction,
using just slightly more than two equivalents of 3, monocoupled proucts were
identified by NMR spectroscopy, yet no free alkyne, from unreacted 3 was
detected. It is likely that under
the reaction conditions small amounts of 2 are being oxidatively homocoupled to form a
bispyridyl butadiyne byproduct. This would not be a problem, except for the
fact that it is not possible to separate the monocoupled material from the
desired decoupled product, 4. It was
possible to take the mixture on to product by reacting the mixture with
additional 3. It is likely that this will interfere
with our ability to synthesize asymmetric analogues of 6. Deprotection of intermediate 4 was achieved with TBAF to give
compound 5.
After workup, 5
was found to be of satisfactory purity by 1H NMR so that no further
purification was necessary.
Oxidative coupling of the terminal alkynes afforded annulene 6 in 30 % yield from 4. This reaction was somewhat problematic in the isolation of
the final product, 6. After the column was
complete, it was clear that the mass balance was not close to that
expected. Flushing the column
recovered more of the product, although less pure than the first. It is clear that more work is needed on
the purification of these compounds. This research has had a
significant impact on all who participated in the project, as well as the PI,
and other students who were not specifically involved in this area. Through presentations and the
experiments themselves, we all learned a great deal with respect to handling
these crown compounds. The summer
had a significant impact on the PI with respect to the desired to continue
working and expanding the research in this area. Students involved in the project benefited from the
experience and were motivated to seek post graduate training in chemistry, one
now having gone to graduate school and a second who has indicated that she now
intends to go to graduate school.