44563-AC4
A Novel System for Production of 1,3-Propanediol Renewable Resources: Characterization of a B12-Independent Glycerol Dehydratase System
Progress Towards Aim 1. - Expression of the B12-Independent Glycerol Dehydratase System in A. vinelandii. In our first progress report, we described results for the expression and subsequent affinity purification of the GD-AE in both E. coli and A. vinelandii. Briefly, for expression of the GD-AE in E. coli, the enzyme was only expressed to detectable levels under aerobic growth conditions and activity had to be reconstituted through the rebuilding of the catalytic [4Fe-4S] cluster. It was further proposed in the first progress report that utilization of the DadhE strain might improve expression levels under both aerobic and anaerobic growth conditions as this gene had been proposed to represent an “inactivase” for pyruvate formate lyase activating enzyme. With respect to this proposal and consistent with recent reports, we have since tried expression of the GD-AE during anaerobic growth in E. coli using the DadhE E. coli strain and saw no significant improvement on the stability of the GD-AE.
Recombination of the GD-AE and GD genes into the chromosome of A. vinelandii did result in a measurable level of activity in the crude extract when S-1,2-propanediol (0.6 + 0.2 mmol min-1 mg-1) and glycerol (0.8 + 0.2 mmol min-1 mg-1) were used as substrates for the GD. Activity for the GD-AE was not very stable in the crude extract and was lost over time or during further affinity purification of the enzyme. At the time of the first report, the mechanism of degradation in vivo, in cell extracts, and during purification was unclear. We have been able to produce the GD-AE and biochemically characterize it following a reconstitution procedure as described in our previous report. Further characterization of the system is described in the following section.
Since our original report we have reconsidered the recombination reaction due to the high GC content of A. vinelandii in contrast to that of C. butyricum (native source of the genes). A. vinelandii is GC rich with an average GC content of over 60% for most open reading frames. This is in contrast to the GC content of the GD-AE gene which is 31 %. In order to further facilitate the production of an active GD-AE and GD system under aerobic growth conditions in A. vinelandii we have subsequently codon-optimized the dhaB1 (GD) dhaB2 (GD-AE) and yqhD (3-HPA reductase) genes for expression in A. vinelandii. The codon-optimized genes for dhaB1, dhaB2, and yqhD were then inserted into a vector contain homologous regions for the nif operon such that recombination leads to insertion in place of the nifH, nifD, and nifK genes respectively. We are currently screening over 30,000 clones and anticipate that this approach will significantly improve the expression and stability of the activity of the system in vivo.
Progress Towards Aim 2. – Characterization of the GD-AE and the GD-(GD-AE) Complex. In our first progress report we provided spectroscopic evidence for two additional [4Fe-4S] clusters in the GD-AE. We have since performed a careful iron analysis using colorimetric assays and ICP-MS for iron determination in the aerobically isolated and reconstituted GD-AE. If the data for both experiments are averaged we detected 14.63 + 1.55 mole of iron per mole of protein in samples of GD-AE that was anaerobically expressed and isolated. It is clear that we need to try and increase the stability of the additional Fe-S clusters in the GD-AE.
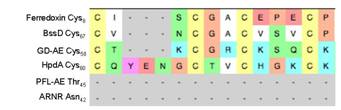
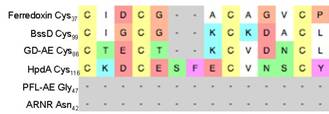
Figure 1. Sequence alignments of Ferredoxin and several radical SAM activating enzymes.
An alignment of the peptide sequence around the proposed “ferredoxin like” clusters in the GD-AE and ferredoxin is shown in Figure 1. Previous work with ferredoxins has shown that a proline residue on the C-terminal side of the CXXC motif is essential for stability of the protein2. We are therefore using site-directed mutagenesis to change the lysine and leucine residues found in this position of GD-AE sequence (See Figure 1) to proline. We anticipate that the K69P and L97P variants of the GD-AE will provide a key step towards stabilizing the GD-AE in the biochemical assay and improving the crystal quality of the (GD-AE)-R782KGD complex.
The broader implications of this work are clear. A future commodity chemicals market will depend more and more on taking advantage of the metabolic diversity found in Nature and will depend less and less on petroleum and natural gas cracking. Completion of our specific aims will allow industry to exploit the ever-growing diversity of this enzyme superfamily.
References.
1. Jacobson, M.R., Cantwell, J.S. & Dean, D.R. J Biol Chem 265, 19429-33 (1990).
2. Quinkal, I., Davasse, V., Gaillard, J. & Moulis, J.M. Protein Eng 7, 681-7 (1994).