

45955-GB5
Bonding and Electronic Structure of Reconstructed Surface Alloys
 Using
the funding provided by this grant, we have investigated the electronic
structures of Al/Pd(100) and Pd/Cu(100), two surface alloy systems that undergo
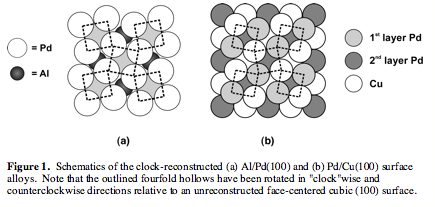
a similar "clock"-type reconstruction (Figure 1) upon formation. As
noted in our proposal, surface alloys are materials that consist primarily of a
single elemental metal, but which have a bimetallic surface composition that is
only a few atomic layers in thickness. Our studies of these two systems were
carried out using the Fenske-Hall band structure method, which is an
approximate Hartree-Fock type method. Using the results of these calculations,
we have been able to relate the optimization of the bonding in the first few
atomic layers of a surface alloy to the experimentally observed displacements
of its surface- and second-layer atoms.
Using
the funding provided by this grant, we have investigated the electronic
structures of Al/Pd(100) and Pd/Cu(100), two surface alloy systems that undergo
a similar "clock"-type reconstruction (Figure 1) upon formation. As
noted in our proposal, surface alloys are materials that consist primarily of a
single elemental metal, but which have a bimetallic surface composition that is
only a few atomic layers in thickness. Our studies of these two systems were
carried out using the Fenske-Hall band structure method, which is an
approximate Hartree-Fock type method. Using the results of these calculations,
we have been able to relate the optimization of the bonding in the first few
atomic layers of a surface alloy to the experimentally observed displacements
of its surface- and second-layer atoms.
The Al/Pd(100) Surface Alloy. To analyze the electronic structure of the Al/Pd(100)
surface alloy, we employed a stepwise theoretical analysis involving bulk Pd, a
clean Pd(100) surface, a hypothetical unreconstructed Al/Pd(100) surface alloy,
and two different clock reconstructed surface alloys (Scheme 1). This analysis
allowed us to correlate features of the electronic structures of these systems
with existing knowledge of 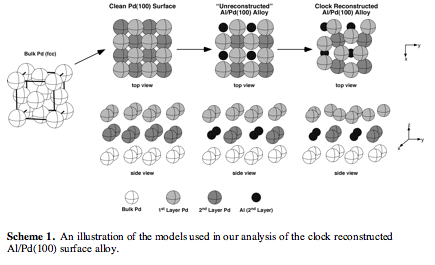 their physical structures.
their physical structures.
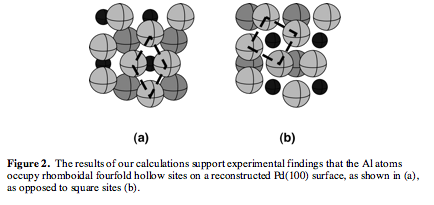
An examination of the bonding in clean Pd(100) supports the experimental observation that aluminum atoms more readily substitute for second layer atoms in Pd(100), as the bonding environments of the second layer Pd atoms are generally weaker than those of top layer Pd. By investigating the electronic structure of an "unreconstructed" Al/Pd(100) surface alloy, it was determined that the substitution of electron-poor aluminum atoms for half of the relatively electron-rich palladium atoms within the second layer provides the surface with a means of depopulating some of the occupied Pd-Pd antibonding states that exist on clean Pd(100). Reconstruction into a clock configuration further stabilizes the surface alloy by simultaneously reinforcing the Pd-Pd bonding within the surface plane, via the creation of a new interaction, and by strengthening the Pd-Al bonding between the first and second atomic layers. Lastly, by comparing two different models for clock reconstructed Al/Pd(100), we provided confirmation of the experimental data which suggests that the more likely structure for the surface alloy is one in which aluminum atoms substitute for second-layer palladium atoms that occupy sites directly beneath the rhomboidal surface sites (Figure 2).
 The Pd/Cu(100) Surface Alloy. We have also applied a stepwise analysis of the
reconstructed Pd/Cu(100) surface alloy; however, this system has proven
considerably more difficult to interpret than the Al/Pd(100) surface alloy.
Our difficulties in identifying the optimal bonding interactions in the
Pd/Cu(100) system may be due to the fact that both palladium and copper are
transition metals. In the Al/Pd(100) system, aluminum, which is a main group
metal, alloys with palladium, and the resulting Al-Pd bonds exhibit some slight
covalent character. The Pd-Cu bonds in Pd/Cu(100) are more purely metallic in
nature than Al-Pd bonds. Since approximate Hartree-Fock methods only generate
information about bonding in terms of orbital overlap, the results of our
Fenske-Hall calculations on the Pd/Cu(100) alloy have proven significantly more
difficult to interpret. Despite this challenge, we are currently working on
organizing our results in a manuscript to be submitted to Surface Science.
The Pd/Cu(100) Surface Alloy. We have also applied a stepwise analysis of the
reconstructed Pd/Cu(100) surface alloy; however, this system has proven
considerably more difficult to interpret than the Al/Pd(100) surface alloy.
Our difficulties in identifying the optimal bonding interactions in the
Pd/Cu(100) system may be due to the fact that both palladium and copper are
transition metals. In the Al/Pd(100) system, aluminum, which is a main group
metal, alloys with palladium, and the resulting Al-Pd bonds exhibit some slight
covalent character. The Pd-Cu bonds in Pd/Cu(100) are more purely metallic in
nature than Al-Pd bonds. Since approximate Hartree-Fock methods only generate
information about bonding in terms of orbital overlap, the results of our
Fenske-Hall calculations on the Pd/Cu(100) alloy have proven significantly more
difficult to interpret. Despite this challenge, we are currently working on
organizing our results in a manuscript to be submitted to Surface Science.
Publications, Presentations, and Impact. To date, the results of our studies funded by the PRF have led to a publication in the journal Surface Science, an undergraduate poster presentation at the 233rd Annual American Chemical Society National Meeting in Chicago, IL (March 2007), and two poster presentations at local undergraduate research symposia. Furthermore, this research project has provided an undergraduate student with a year and a half of significant research experience in computational chemistry. Upon graduation from UW-La Crosse, this student went on to pursue graduate studies in theoretical chemistry at UW-Madison.
This grant has significantly impacted the PI's career in a positive way, as well. It has provided strong evidence to her department that she is capable of setting up and maintaining an active program in computational chemical research involving undergraduate students. It has also allowed her to devote her summers to research, which is particularly beneficial to someone at a primarily undergraduate institution with higher teaching loads during the regular academic year.