

46803-AC10
Heterometallic Oxides/Organics For Investigation of Visible-Light Photocatalysis
The project has focused on two main areas that center around two synthetic approaches for achieving a new molecular-level structural control over the photocatalytic properties of materials: (1) incorporation of organic ligands into heterometallic oxides to enable a finer molecular-level structural control, and (2) molten-salt flux reactions for the controlled growth of metal-oxide particle sizes from nanometers up to micrometers. These investigated materials are those previously proposed in my research plans and contain combinations of transition metals with electron configurations that create an optimal band-energy profile for visible-light absorption and resulting photocatalytic activity.
 Heterometallic-Oxide/Organic Hybrid Solids:
Ligand Effects on the Structures and Properties of Metal-Oxides. Understanding the effects of ligand
coordination geometries and sizes on the internal structures of metal oxides is
essential for optimizing their optical and photocatalytic properties at the
molecular level. We have been able to
significantly expand our hydrothermal synthetic efforts by using a number of
selected types of coordinating ligands, and which have resulted in many new
heterometallic-oxide/organic solids (i.e., MM'OL; M/M' = transition metals with
d0 and d10 electron configurations; L = coordinating
ligand) in the Cu/Re, Ag/Re, Cu/V and Ag/V systems. In the vanadate systems, a series of new hybrid solids were
prepared that are comprised of silver-vanadate layers pillared by organic
ligands (bpy, dpa, and pzc). These have
led to a new understanding, for example, of the effect of ligand length on the
amount and reversibility of water absorption into their structures (e.g.,
[Ag(bpy)]4V4O12∙2H2O versus
[Ag(dpa)]4V4O12∙4H2O). The coordination geometry of the ligand was
also found to impact the network dimensionality and resulted in a significant
variability of the optical bandgap sizes from ~2.45 – 2.95 eV, with the
smallest bandgap size of 2.45 eV achieved for Ag4(pzc)2V2O6. Further, the latter material is the first
known hybrid-oxide/organic that has been found to exhibit photocatalytic
activity under visible-light irradiation.
The rhenate hybrids are also proving to be an extremely rich system
where the organic ligand can be used to vary the dimensionality of the
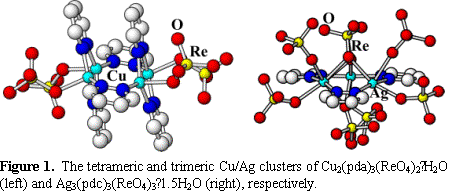
organic/inorganic components, such as from isolated trimeric and tetrameric
cluster units in Ag3(pdc)3(ReO4)3∙1.5H2O
and Cu2(pda)3(ReO4)2∙H2O
to layered structures in M(bpy)ReO4 (A = Cu, Ag), and network types
of structures in Cu(bpy)2ReO4∙ ˝H2O and
Ag(id)2ReO4.
Their optical property measurements are currently underway, and are
enabling new investigations into the effects of coordination environments and
framework dimensionalities on the measured bandgap sizes and types.
Heterometallic-Oxide/Organic Hybrid Solids:
Ligand Effects on the Structures and Properties of Metal-Oxides. Understanding the effects of ligand
coordination geometries and sizes on the internal structures of metal oxides is
essential for optimizing their optical and photocatalytic properties at the
molecular level. We have been able to
significantly expand our hydrothermal synthetic efforts by using a number of
selected types of coordinating ligands, and which have resulted in many new
heterometallic-oxide/organic solids (i.e., MM'OL; M/M' = transition metals with
d0 and d10 electron configurations; L = coordinating
ligand) in the Cu/Re, Ag/Re, Cu/V and Ag/V systems. In the vanadate systems, a series of new hybrid solids were
prepared that are comprised of silver-vanadate layers pillared by organic
ligands (bpy, dpa, and pzc). These have
led to a new understanding, for example, of the effect of ligand length on the
amount and reversibility of water absorption into their structures (e.g.,
[Ag(bpy)]4V4O12∙2H2O versus
[Ag(dpa)]4V4O12∙4H2O). The coordination geometry of the ligand was
also found to impact the network dimensionality and resulted in a significant
variability of the optical bandgap sizes from ~2.45 – 2.95 eV, with the
smallest bandgap size of 2.45 eV achieved for Ag4(pzc)2V2O6. Further, the latter material is the first
known hybrid-oxide/organic that has been found to exhibit photocatalytic
activity under visible-light irradiation.
The rhenate hybrids are also proving to be an extremely rich system
where the organic ligand can be used to vary the dimensionality of the
organic/inorganic components, such as from isolated trimeric and tetrameric
cluster units in Ag3(pdc)3(ReO4)3∙1.5H2O
and Cu2(pda)3(ReO4)2∙H2O
to layered structures in M(bpy)ReO4 (A = Cu, Ag), and network types
of structures in Cu(bpy)2ReO4∙ ˝H2O and
Ag(id)2ReO4.
Their optical property measurements are currently underway, and are
enabling new investigations into the effects of coordination environments and
framework dimensionalities on the measured bandgap sizes and types.
Flux Synthesis of Metal-Oxides Particles: Effects of Particle Growth on Optical and Photocatalytic Properties. At a larger size-scale regime, the molten-salt flux growth of heterometallic-oxide particles (e.g., MM'O) in the Ag/Nb and Cu/Nb systems has been investigated towards achieving a finer control over particle sizes and morphologies. In an initial feasibility study, the (110) layered perovskite La2Ti2O7 was first selected to study the effects of flux synthetic conditions on the underlying particle features and surfaces that govern its high photocatalytic activity. Platelet-shaped particles with size dimensions of ~500 – 6,000 nm in width and <100nm in thickness could be prepared in a Na2SO4/K2SO4 flux at 1100 oC in short reaction times of 1 – 10h. The particle sizes can be controlled within these ranges by adjusting the reaction time and flux amounts, and exhibited high photocatalytic rates for hydrogen formation in both aqueous methanol (~55-140 μmol H2 h-1g-1) and in deionized water solutions (~31 μmol H2 h-1g-1).
 Our studies have been able
to reveal that the crystallite edges and (010) and (001) crystal faces play a
key role in its photocatalytic reactivity.
We also expanded these investigations to the AgNbO3, CuNbO3,
and CuNb3O8 solids, to investigate whether flux
preparations can similarly ‘activate' the surfaces of these solids that have
visible-light bandgap absorption. In
ongoing work, the rapid single-step synthesis of AgNbO3 particles
with sizes of ~500 – 5,000 nm can be obtained in a Na2SO4
flux at 900 oC in only 1 – 10 h.
Smaller particle-size distributions are found for increasing amounts of
flux and longer reaction times. Their
photocatalytic activity in visible light has been correlated with the formation
of ~20 – 50 nm ‘nano-terraced' surface features, and which are absent in the
non-active AbNbO3 samples prepared by solid-state methods. The highest observed photocatalytic rates
for AgNbO3 particles of ~60 μmol H2 h-1g-1
are obtained in an aqueous methanol solution for the largest particle sizes,
but which have a larger amount of the ‘nano-terraced' surfaces features. Currently, our studies on AgNbO3
have shown that while particle size, in general, can impact the amount of total
surface area of a metal oxide, it is even more critical to closely assess the
relative concentration of the particles' surfaces that are active.
Our studies have been able
to reveal that the crystallite edges and (010) and (001) crystal faces play a
key role in its photocatalytic reactivity.
We also expanded these investigations to the AgNbO3, CuNbO3,
and CuNb3O8 solids, to investigate whether flux
preparations can similarly ‘activate' the surfaces of these solids that have
visible-light bandgap absorption. In
ongoing work, the rapid single-step synthesis of AgNbO3 particles
with sizes of ~500 – 5,000 nm can be obtained in a Na2SO4
flux at 900 oC in only 1 – 10 h.
Smaller particle-size distributions are found for increasing amounts of
flux and longer reaction times. Their
photocatalytic activity in visible light has been correlated with the formation
of ~20 – 50 nm ‘nano-terraced' surface features, and which are absent in the
non-active AbNbO3 samples prepared by solid-state methods. The highest observed photocatalytic rates
for AgNbO3 particles of ~60 μmol H2 h-1g-1
are obtained in an aqueous methanol solution for the largest particle sizes,
but which have a larger amount of the ‘nano-terraced' surfaces features. Currently, our studies on AgNbO3
have shown that while particle size, in general, can impact the amount of total
surface area of a metal oxide, it is even more critical to closely assess the
relative concentration of the particles' surfaces that are active.