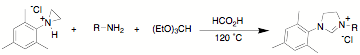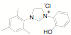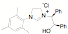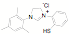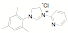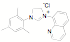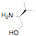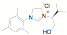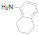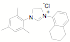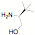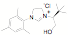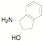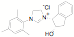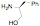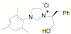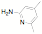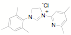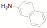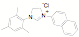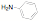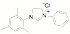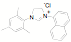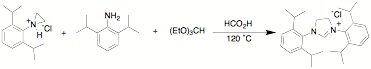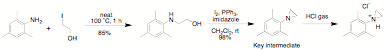46269-AC1
A Modular Approach to Chiral NHC Ligands and Their Use in Asymmetric Catalysis
The discovery of new asymmetric catalysts is often limited by the availability of new ligands. Consequently the development of chemistry that provides access to new ligand types is critical. This work focuses on the development of building blocks and reactions for the construction of a ligand type that has been shown to provide metal complexes possessing unique reactivity, N-heterocyclic carbene (NHC) ligands.
We have developed a one
step approach toward the synthesis of unsymmetrical NHC ligands. The reaction
of a protonated aziridine with a primary amine has been used to synthesize a
series of salts that are common precursors for NHC metal complexes. This
approach has been used to synthesize a variety of new unsymmetrical NHC
ligands. Fifteen of the ligands have been synthesized from the mesitylene
aziridine and one has been prepared from the 2,6-diisoproply aziridine. These
aziridines can be reacted with aromatic and aliphatic amines in the presence of
triethylorthoformate to form the desired NHC precursor. Thus far we have only
examined the mesitylene and
2,6-diisopropyl aziridines. These examples were selected in part because they
represent two of the more hindered examples. Since they work well it is highly
likely that other less hindered examples will also be useful. The yields for
aziridine openings are highter for aromatic amines than for the aliphatic
amines that have been attempted. This approach is compatible with a number of
potentially reactive groups. NHC ligands have been synthesized in one step from
amino alcohols, amino pyridines and amino thiols without the need to protect
the alcohol, thiol or pyridine groups.
Table 1
Entry R Yield Product Entry R Yield Product 1 29% 9 65% 2 50% 10 >30% 3 72% 11 85% 4 32% 12 60% 5 31% 13 56% 6 35% 14 94% 7 77% 15 76% 8 82%
The key to this process
being an expedient approach to a variety of different NHC ligands is the
availability of the aziridine starting material. We have synthesized multi gram
quantities of the necessary aziridines by reaction of the appropriate amine
with 2-iodoethanol followed by reaction with iodine and triphenylphosphine
(Scheme qq). We have scaled up the
synthesis of a number of these ligands and are in the process of screening them
against a number of different reactions, both metal and nucleophilicly
catalyzed reactions. The reactions that will be examined first are, the rhodium
catalyzed 1,2-addition of boronic acids to aldehydes, the metathesis reaction
of meso trienes to obtain asymmetric products. We will also examine rhodium
complexes with these ligands as catalysts for the 4+2+2+ reaction we have
discovered.