
Back to Table of Contents
44703-GB3
Amphiphilic Metal Complexes: A Novel Approach to Aqueous Nanosystems with Catalytic Function
Juan Noveron, University of Texas at El Paso
The development of new metal complexes with alkyl (CnH2n+1, n>10) and
oligoether (CH2CH2O-)4 components that
allow them to self-organize into nanoscale supramolecular phases in water while
retaining their active coordination chemistry was the focus of our research.
The new lipid metal complexes developed in this project pose a new avenue for
further innovation for green catalysis design and the development of functional
materials in water that may play a role in the environmental remediation,
biomass conversion and in the manufacture of pharmaceuticals.
1. Lipid
Metal Complexes. We synthesized and characterized seventeen amphiphilic
ligands (1-17), Figure 1. The lipid groups introduced self-assembly
properties to these molecules and their corresponding metal complexes in water.
They allowed us to investigate the intrinsic chemical and physical properties
of their corresponding metal complexes. In this final report, we highlight some
of the most interesting results that we obtained during the Grant period.
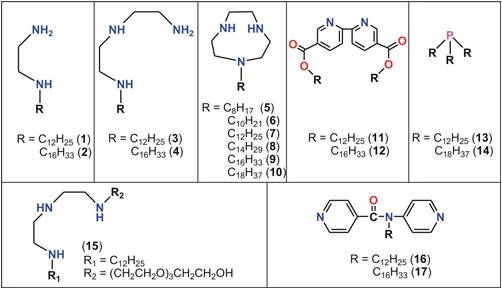
Figure
1.
Lipid ligand systems synthesized (1-17).
a. Novel Copper(I, II) lipid complexes with
dioxygen-activation functions. Lipid Cu(I) and (II) complexes of ligands 1, 3,
7, 11 and 15 were
synthesized and characterized with EXAFS, electrospray mass spectrometry
(ESMS), and UV-vis spectroscopy. The mononuclear Cu(I) complexes of these
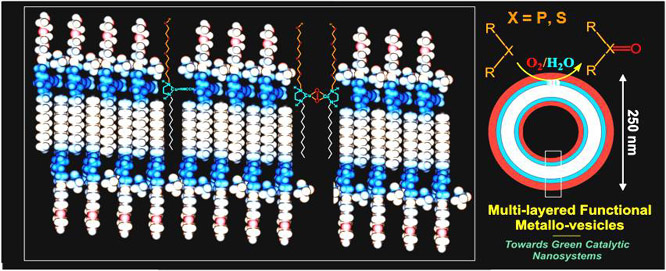
ligands formed vesicles in water. The lipid ligand 15 generated multi-layered vesicles, Figure 2a, in which the Cu(I)
sites are located within a hydrophobic environment that stabilized the copper m-oxo species. The [(15)2Cu2(m2-O)2] was observed in
EXAFS, Figure 2b. The vesicles generated from Cu(I) complexes of 1, 3,
7, and 11 did not generate
kinetically stable m-oxo species in water. The oxidative potential of [(15)2Cu2(m2-O)2] was investigated
using triphenylphospine
(Ph3P) as the substrate. We found that [(15)2Cu2(m2-O)2] mediates the
oxidation of Ph3P
(55% yield) to the corresponding phosphine oxide (Ph3P=O) in water
at 4 oC, Figure 2c. The control revealed essentially no oxidation of
Ph3P in the absence of the metal under similar conditions. The
use of the EXAFS line at the Stanford Synchrotron Radiation Laboratory (SSRL) was used to collect X-ray
spectroscopy data to study the coordination chemistry of seventeen metal lipid
complexes during the period of the Grant. Time-lapse EXAFS was used to follow
the oxo-transfer reaction from dioxygen to phosphine model substrates mediated
with Cu(I) lipid complexes, Scheme 1.
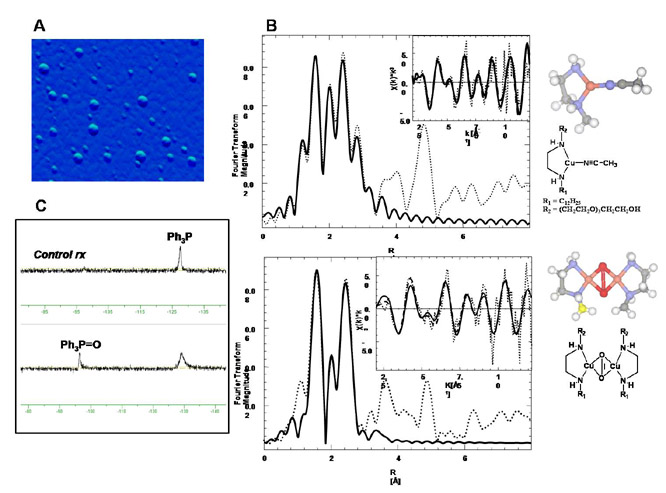
Figure
2.
(A) AFM scan of [Cu(15)(ACN)]+
vesicles in water. (B) Above: Fourier transformed EXAFS of [Cu(15)(ACN)]+
(dotted line) and fitting (solid line). Below: Cu-lipid complex upon reaction
with dioxygen at 77 K. (C) 31P NMRs of the products of the reactions
of [(15)2Cu2(m2-O)2] with
triphenylphosphine in water.
Scheme 1. Concept
illustration of this reaction.
Similarly, vesicles prepared in water with
[Cu(II)(10)(OTf)2], where
OTf = trifluoromethanesulfonate, exhibit oxidase activity with
pyridyl-containing secondary alcohols such as 4,4'-dipyridylmethanol and
catalyze the two-electron oxidation to the corresponding ketone, Figure 3.
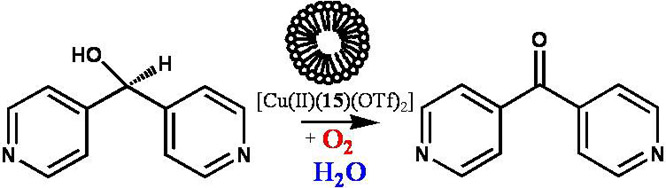
Figure 3. Oxidase
activity of vesicles of [Cu(II)(10)(OTf)2]
in water.
b. Lipid Cu(II) and Zn(II) complexes with
hydrolytic catalytic properties in water. We prepared coordinatively unsaturated
Cu(II) and Zinc(II) complexes of ligands 3,4,7, 12, and 15 and prepared their corresponding
metallo-liposomes in water. These lipid complexes catalyze the hydrolysis of
carboxylic esters and phosphate esters at mild conditions (pH 7.1 and
22 oC), Figure 4. Hydrolytic function was probed with the model
substrates p-nitrophenylacetate and p-nitrophenylphosphate.
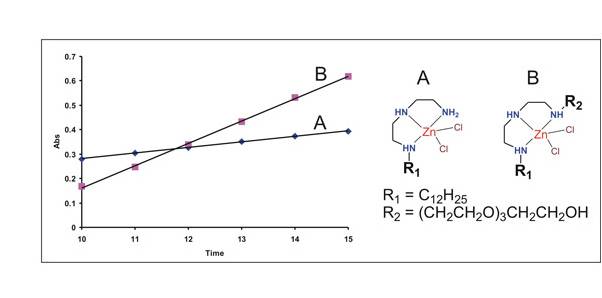
Figure 4. Catalytic
hydrolysis of p-nitrophenylacetate by lipid Zn(II) complexes A and B in water
(pH 7, 22 oC). (Time scale is in min.)
c. Lipid-metal
Coordination Networks and their Self-assembly Properties in Water. We discovered that reactions
of [Cu(II)(7)(OTf)] with
4,4'-trimethylenedipyridyl (4,4-TDP) generate one dimensional coordination
networks that self-assemble in water into nanoscopic toroidal structures. Using
X-ray crystallography, we crystallized the non-lipid model of this complex,
Figure 5.
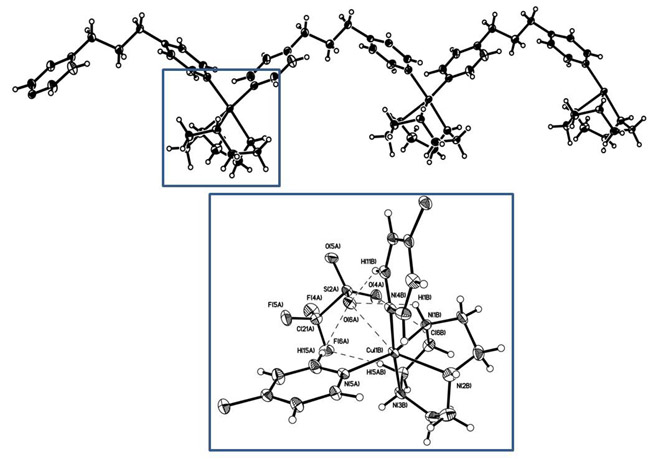
Figure 5. Crystal
structure of the one-dimensional coordination Cu(II) network and close-up of
Cu(II) site showing interactions with ligands and the -OTf counter ion. When the lipid coordination network [Cu(II)(7)( 4,4-TDP)] (OTf)2 is
placed in water (1 mM, 25 oC), it folds into toroidal-like
structures, Figure 6. Molecular weight determinations with dynamic light
scattering (DLS) are currently undergoing, and attempts to control the polymer
size with capping coordinating molecules is being explored. These materials
could lead to robust catalytic systems that operate in water.
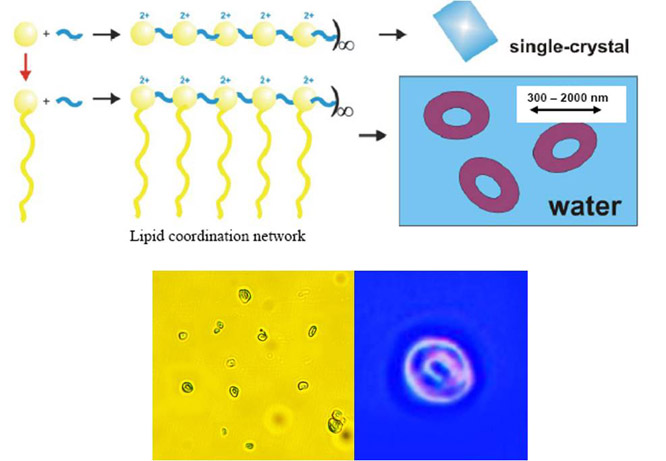
Figure 6. Above: Scheme of
lipid coordination polymers. Below: Optical microscope images of toroidal
structures generated from the lipid-directed folding of the 1-D coordination
network of [Cu(II)(7)(
4,4-TDP)]n (OTf)2n in water.
d. Lipid
Pt(II) and Pd(II) complexes and their self-assembly properties in water.
We also prepared the Platinum(II) and Palladium(II) lipid complexes with
ligands 13 and 14. Two examples are
displayed in Figure 7 and 8. Lipid metallacycles of Pt(II) generated micelles
in water.
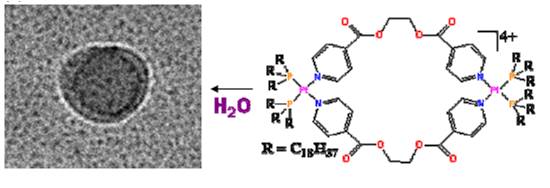
Figure 7. Cryo-TEM image of
dinuclear lipid Pt(II) complex with ligand 14.
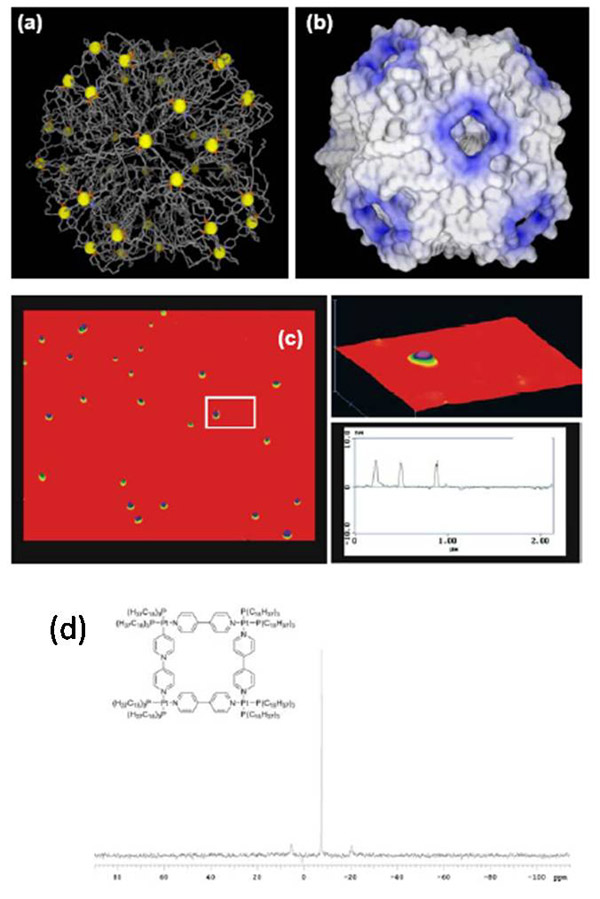
Figure 8. Tetranuclear Pt(II)
macrocyclic lipid complex self-assembled into micelles in water. (a) Computer
model (b) surface electronic potential. (c) AFM scans on mica. (d) 31P
NMR.
Back to top










