41360-B4
Ring Strain and Antiaromaticity: Their Effect on the Generation and Reactivity of Enolates in Small-Ringed Ketones
The fundamental importance of enolates as intermediates in the formation of carbon-carbon bonds is obvious when one considers the amount of space devoted to this topic in Organic Chemistry textbooks. An area of enolate chemistry that has not received as much attention is the effect of ring strain on the generation and reactivity of enolates in strained ring cyclic ketones. The affect of the ring and ring strain is manifested in several different ways; a) Increased s-character in the alpha C-H bond, increasing acidity of the C-H bond. b) Upon ionization, rehybridization of the alpha-carbon to sp2-hydbridization should destabilize the transition state and the enolate relative to the starting material. c) The ring decreases the rotational degrees of freedom of the alpha-carbon resulting in the alpha protons being better aligned with the pi-bond of the carbonyl group. The first two effects should compete against one another making it difficult to predict which effect may predominate during the ionization of cyclic ketones. The results of these studies will provide results that could be correlated to many systems, including enzymatic. For example, determining the role of ring strain in all phases of enolate production will have important implications in our understanding of the mechanistic imperatives for the generation of enolates. This is particularly important in light of the regularity with which the induction of strain is implicated as being catalytically important in enzymatic catalysis. The ketones in which we are interested have varying degrees of strain and the α-protons have reduced mobility due to the ring.
i) Determining the pKa of
Benzocyclobutenone (1): Previously, we had determined the rate of the
quinuclidine-catalyzed (pKBH
= 11.5) deprotonation of 1 to be kB =
7.2 x 10-6 M-1 s-1in D2O, at 25oC
and I = 1.0 (KCl). In addition the
rate of the 3-quinuclidinol (pKBH
= 10.0) catalyzed reaction was found to be kB = 4.8 x 10-7
M-1s-1 in D2O, at 25oC and I = 1.0 (KCl). This project is currently
in hiatus waiting for a new volunteer. ii) Reactivity
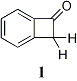
Studies of Substituted Benzocyclobutenone Derivatives: An undergraduate, Rick Yarbrough,
continued our work in this area until his graduation in the Spring
of 2008 (Currently employed by Abbott Laboratories). He has synthesized a
number of aromatic substituted derivatives, using the scheme above, and
purified the methoxy, and chloro derivatives. The methyl derivative proved to
be more difficult to synthesize as two regioisomers
resulted. This problem has been overcome via
isolation using preparative HPLC and, at least, one of the isomers has now been
purified. iii) Determination
of the pKa of Cyclobutanone: We have performed deuterium
incorporation studies on cyclobutanone and based upon the second-order rate
constants for general-base catalyzed determined the pKa of cyclobutanone to be 20.0. This aspect of the
project then began to develop as continued our deuterium incorporation studies
with the lesser strained cyclopentanone system. Previous to our investigations
of cyclopentanone, all deuterium incorporation studies involving enolate
intermediates relied heavily on results from studies involving acetone.
However, the rate constants for enolate formation in cyclobutanone were not
significantly different than those obtained for acetone. This led to questions
concerning the effect of the carbocycle restricting the orientation of the a-protons with regard to
the carbonyl group. The pKa
of cyclopentanone was estimated to be 18.5, which is considerably more acidic
than both acetone and cyclobutanone. These results led to further difficulties
because in all cases cyclopentanone reacted more quickly than cyclobutanone.
Previous studies found that the relative rate of enolization were C4 > C5
> C6 > C7 ~ 4-heptanone or C4 > C5 > C8 > C7 > C6. We were beginning to see trends
in the reactivity of these cyclic compounds that were beginning to shed light
on the role of both ring-strain and the effects of the carbocycle restricting
C-C rotational freedom. We have expanded this project to include cyclohexanone
and 3-pentanone. Early results indicate that cyclohexanone is not as reactive
as cyclopentanone but is more reactive than cyclobutanone. iv) Effect of Increasing Ring
Strain on the Reactivity of a-Protons: The synthesis of bicyclo
 The
proposed studies had four fundamental parts and each of these sections will be
addressed independently.
The
proposed studies had four fundamental parts and each of these sections will be
addressed independently.