

43176-AC4
In Search of Aryloxenium Ions
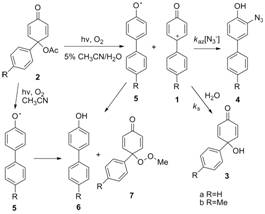 Aryloxenium ions 1
are reactive intermediates that are isoelectronic
with the better known arylcarbenium and arylnitrenium ions. They are proposed to be involved in
synthetically and industrially useful oxidation reactions of phenols. However,
mechanistic studies of these intermediates are limited. Until recently, the
lifetimes of these intermediates in solution and their reactivity patterns were
unknown. Previously we reported that the 4-aryl ions 1 were generated by
hydrolysis of 4-aryl-4-acetoxy-2,5-cyclohexadienones, 2.
The ions were indirectly detected by 18O-labeling studies in 18O-H2O,
common ion effects, and the formation of the azide
adducts 4 at the expense of the hydrolysis products 3 under conditions in which the
decomposition rates of the precursors were independent of [N3-].
Laser flash photolysis (LFP) of 2b
in the presence of O2 in aqueous solution leads to two reactive
intermediates with λmax 360
nm and 460 nm, respectively, while in pure CH3CN, only one species
with λmax 350 nm is
produced. The intermediate with λmax
460 nm was previously identified as 1b
based on direct observation of its decomposition kinetics in the presence of N3-,
comparison to azide ion trapping results from the
hydrolysis reactions, and photolysis reaction products (3b). The agreement between the calculated (B3LYP/6-31G(d)) and observed time resolved resonance Raman (TR3)
spectra of 1b further confirms its identity. The second intermediate
with λmax 360 nm (350 nm
in CH3CN) has been characterized as the radical 5b, based on its photolytic generation in the less polar CH3CN,
and on isolated photolysis reaction products (6b and 7b). Only the
radical intermediate 5b is generated by photolysis in CH3CN,
so its UV-vis spectrum, reaction products, and decay
kinetics can be investigated in this solvent without interference from 1b.
In addition, the radical 5a was generated by LFP of 2a, and was
identified by comparison to a published UV-vis
spectrum of authentic 5a obtained under similar conditions. The
similarity of the UV-vis spectra of 5a and 5b,
their reaction products, and the kinetics of their decay confirm the assigned
structures. The lifetime of 1b in
aqueous solution at room temperature is 170 ns. This intermediate decays with
first-order kinetics. The radical intermediate 5b decomposes in a biphasic manner, with lifetimes of 12 μs and 75 μs. The decay
processes of 5a and 5b were successfully modeled with a kinetic
scheme that included reversible formation of a dimer.
The scheme is similar to the kinetic models applied to describe the decay of
other aryloxy radicals.
Aryloxenium ions 1
are reactive intermediates that are isoelectronic
with the better known arylcarbenium and arylnitrenium ions. They are proposed to be involved in
synthetically and industrially useful oxidation reactions of phenols. However,
mechanistic studies of these intermediates are limited. Until recently, the
lifetimes of these intermediates in solution and their reactivity patterns were
unknown. Previously we reported that the 4-aryl ions 1 were generated by
hydrolysis of 4-aryl-4-acetoxy-2,5-cyclohexadienones, 2.
The ions were indirectly detected by 18O-labeling studies in 18O-H2O,
common ion effects, and the formation of the azide
adducts 4 at the expense of the hydrolysis products 3 under conditions in which the
decomposition rates of the precursors were independent of [N3-].
Laser flash photolysis (LFP) of 2b
in the presence of O2 in aqueous solution leads to two reactive
intermediates with λmax 360
nm and 460 nm, respectively, while in pure CH3CN, only one species
with λmax 350 nm is
produced. The intermediate with λmax
460 nm was previously identified as 1b
based on direct observation of its decomposition kinetics in the presence of N3-,
comparison to azide ion trapping results from the
hydrolysis reactions, and photolysis reaction products (3b). The agreement between the calculated (B3LYP/6-31G(d)) and observed time resolved resonance Raman (TR3)
spectra of 1b further confirms its identity. The second intermediate
with λmax 360 nm (350 nm
in CH3CN) has been characterized as the radical 5b, based on its photolytic generation in the less polar CH3CN,
and on isolated photolysis reaction products (6b and 7b). Only the
radical intermediate 5b is generated by photolysis in CH3CN,
so its UV-vis spectrum, reaction products, and decay
kinetics can be investigated in this solvent without interference from 1b.
In addition, the radical 5a was generated by LFP of 2a, and was
identified by comparison to a published UV-vis
spectrum of authentic 5a obtained under similar conditions. The
similarity of the UV-vis spectra of 5a and 5b,
their reaction products, and the kinetics of their decay confirm the assigned
structures. The lifetime of 1b in
aqueous solution at room temperature is 170 ns. This intermediate decays with
first-order kinetics. The radical intermediate 5b decomposes in a biphasic manner, with lifetimes of 12 μs and 75 μs. The decay
processes of 5a and 5b were successfully modeled with a kinetic
scheme that included reversible formation of a dimer.
The scheme is similar to the kinetic models applied to describe the decay of
other aryloxy radicals.