46532-GB4
Synthesis and Characterization of Novel Donor/Acceptor Molecules Based on Propellanes: A Probe of Nonclassical Conjugation
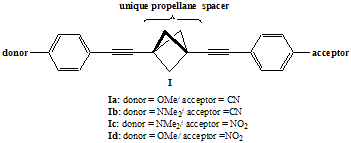
Novel donor/acceptor molecules (I) based on the 1,3-diethynylbicyclo[1.1.1]pentane (diethynylpropellane) spacer are being investigated as a probe of nonclassical conjugation through the propellane unit. The work represents the first experimental study of charge transfer from donor (D) to acceptor (A) chromophores linked by diethynylpropellanes. The propellane unit should lead to substantial charge transfer between the donor and acceptor moieties via through-bond interaction.1 Molecules based on diethynylpropellane spacers bearing different donor and acceptor combinations will be prepared and then characterized by UV-vis and fluorescence spectroscopy to determine the extent of interaction through the propellane spacer.
We have prepared four of the target D/A molecules using
sequential Sonogashira coupling2 of the
appropriate donor or acceptor halobenzenes to the
parent diethynylpropellane, which is available in four
steps from 1,1-dibromo-2,2-bis(chloromethyl)cyclopropane.3 Syntheses of additional targets bearing the SMe donor are in progress.
A series of corresponding p-phenylacetylenes were also prepared,
and their UV-vis spectra compared to those for the D/A
molecules. In general, the target
molecules absorb at longer wavelength than the corresponding p-phenylacetylenes
or the parent diethynylpropellane in the UV-vis, and this is attributed to through-bond interaction mediated
by the propellane spacer. The UV-vis
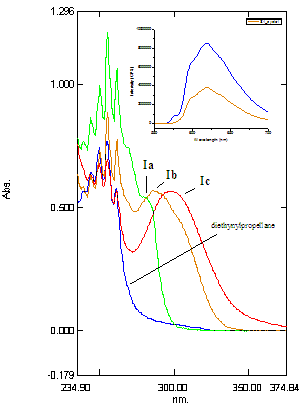
absorption spectra are collected in Fig. 1.
As the strength of the D/A combination increases, the longest wavelength
absorption shifts to higher wavelength for the series in dioxane: Ia (OMe/CN at 284nm) < Ib (NMe2/CN at
288 nm) < Ic (NMe2/NO2 at 297 nm). Such sensitivity to the combination of D/A
groups suggests communication through the propellane
spacer. The absorption spectra exhibit some
solvent dependence, but the behavior is complex. Surprisingly, the CN/OMe
derivative Ia is slightly blue shifted ( < 5 nm) upon going from nonpolar
cyclohexane to polar acetonitrile. Further study of the UV-vis
spectra of these molecules is needed to explain this solvent dependent behavior
and is currently underway.
Figure
1. UV-vis
Absorption spectra for the parent diethynylpropellane,
Ia,
Ib and Ic in dioxane (ca. ~ 10-5M). Inset:
Fluorescence emission spectrum for Ia in pentane, excitation
at 285 nm (blue), 280 nm (orange).
Preliminary fluorescence studies indicate significant charge
transfer fluorescence for the CN/OMe derivative Ia. The molecule exhibits a single, broad
emission at 618 nm (excitation at 285 nm) that does not correspond to the
expected fluorescence of the individual aromatic chromophores. (See inset in Fig. 1.) The emission most likely arises from an intramolecular charge transfer state, and we plan to study
the effect of solvent polarity on the emission wavelength. If the emission is highly solvatochromic,
it will support this prediction. We will
carry out similar studies on other D/A molecules as they are prepared.
Support from the ACS-Petroleum Research Fund has made a
significant impact on the principle investigator's (PI) research program and
has allowed the PI to pursue research in a new area of study. Most importantly, funding has provided
research opportunities for five undergraduate students. The project is challenging and has exposed
students to a variety of synthetic and analytical laboratory techniques. Three students presented their work on this
project at an on-campus symposium highlighting undergraduate research; one
student will present at the regional Argonne Undergraduate Symposium in
November 2008.
The impact of the funded work is further demonstrated by the
fundamental questions it will address regarding nonclassical
conjugation through the propellane spacer. The target molecules appear to exhibit
significant polarization, and the effects of the propellane
unit on the electronic and optical behavior of these systems will be
interesting. Although beyond the present
scope of this work, significant charge transfer interaction may lead to
applications of these units as linkers for electron or energy transfer
processes.
(1) Gleiter, R.;
Pfeifer, K.-H.; Szeimies, G.; Bunz,
U. Angew. Chem., Int. Ed. Engl. 1990, 29, 413-415. (2) (a) Takahashi, S.; Kuroyama, Y.; Sonogashira, K.; Hagihara, N. Synthesis
1980, 627-630. (b) Hundertmark,
T.; Littke, A.F.; Buchwald, S.L.; Fu, G.C. Org. Lett. 2000, 2, 1729-1731. (3) (a) Semmler, K.; Szeimies, G.; Belzner, J. J. Am.
Chem. Soc. 1985, 107, 6410-6411. (b) Lynch, K. M.; Dailey, W. P. Org. Synth.
1997, 75, 98-105 (c) Kaszynski, P.; Michl, J. J.
Org. Chem. 1988, 53, 4593-4594. (d)Levin, M. D.; Kaszynski, P.; Michl, J. Org. Synth. 2000, 77, 249-253.