

46587-G3
Transition-Metal-Mediated Activation of Sulfur Toward C–S Bond Formation
Synthesis, Characterization, and Reactivity of Iridium(I) Complexes Supported by Guanidinato Ligands
Monoanionic nitrogen-donor ligands represent an important class of supporting ligands and have been widely used in the coordination chemistry of transition metals to impart diverse properties and reactivity. Prominent examples include poly(1-pyrazolyl)borate, β-diiminate, aminotroponiminate, and amidinate scaffolds. Since guanidinates are conceptually related to the latter three systems, they may be expected to be similarly well-suited as supports for reactive metal centers. Although complexes of guanidinates are known for many metals and metalloids from across the periodic table, examples involving electron-rich transition metal centers in low oxidation states are limited to only a few di and tetranuclear complexes, which mainly involve coinage metals.
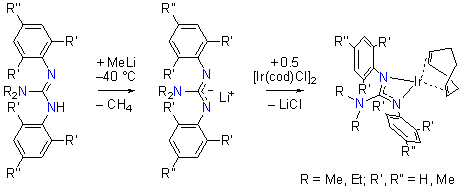
We have synthesized IrI(cod) complexes of a series of bidentate N,N-dialkyl-N',N''-diarylguanidinate anions, ArNC(NR2)NAr–, to explore the coordination ability of this ligand platform to transition metals in low oxidation states and to probe its utility in tuning the reactivity of the coordinated metal center. The neutral guanidines, ArN=C(NR2)NHAr, were readily prepared from the corresponding thioureas, ArHNC(S)NHAr, and the appropriate amines, HNR2, according to literature methods for related compounds. As shown in Scheme 1, treatment with MeLi and subsequent transmetallation using [{Ir(cod)}2(μ-Cl)2] under an inert atmosphere furnished the yellow IrI complexes of the anionic guanidinates, [Ir{ArNC(NR2)NAr}(cod)], in good yields.
Scheme 1. Synthesis of [Ir{ArNC(NR2)NAr}(cod)] Complexes
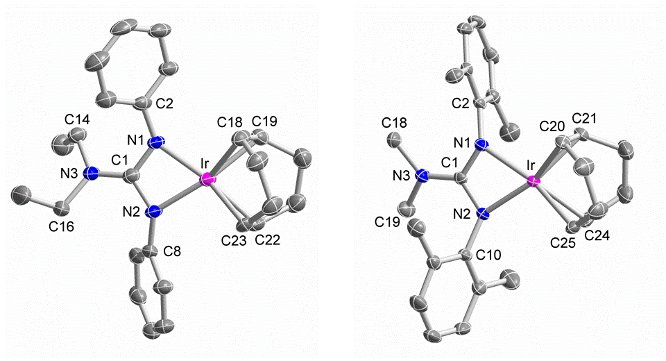
The ligands and complexes were fully characterized by elemental analysis, mass spectrometry, electronic absorption, 1H and 13C NMR spectroscopy, and X-ray crystallography (Figure 1). Detailed inspection of the structural and 13C NMR data of these complexes revealed that the anionic dialkyldiarylguanidinates can still function as strongly donating ligands when bound to the IrI(cod) unit. For example, the structural data indicate significant NR2 lone-pair donation, and the d(13CC=C) resonance signals, which reflect increased Ir-to-cod π back-bonding, suggest that the dialkyldiarylguanidinates may be considered stronger donors than κ2-binding tris(pyrazolyl)borates and β-diketiminates but weaker donors than tris(pyrazolyl)borate-κ3 and cyclopentadienyl ligands.
Figure 1. Molecular structures of [Ir{PhNC(NEt2)NPh}(cod)] (left) and [Ir{(2,6-Me2C6H3)NC(NMe2)N(2,6-Me2C6H3)}(cod)] (right); hydrogen atoms have been omitted for clarity; color key: pink = Ir, blue = N, gray = C.
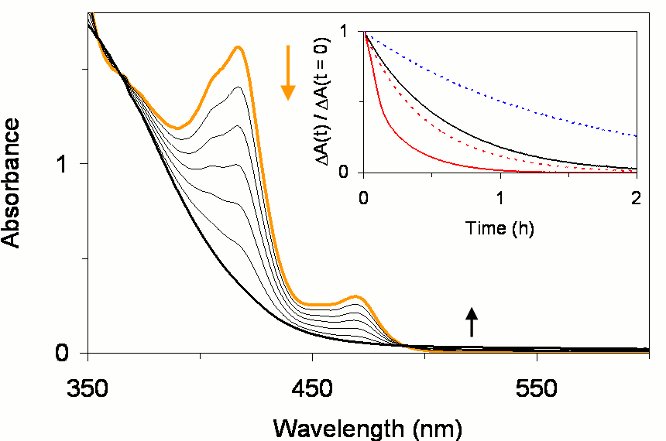
In solution, the [Ir{ArNC(NR2)NAr}(cod)] complexes readily react with dioxygen at ambient temperature and pressure (Figure 2). Mono and di-oxygenated products were identified from the reaction mixtures. A systematic investigation of substituent effects showed that the reaction rates can be altered by the electronic and steric properties of substituents in both amino and amidinato positions of the guanidinato ligand. The new complexes also react with elemental sulfur, and the elucidation of reaction mechanisms and products is currently underway.
Figure 2. Reaction of [Ir{PhNC(NMe2)NPh}(cod)] in toluene with O2 at 0 °C as monitored by electronic absorption spectroscopy. Inset: Time courses of the reactions of [Ir{ArNC(NR2)NAr}(cod)] complexes with O2.
In summary, we have prepared and characterized mononuclear complexes of a low-valent d-block metal center coordinated by didentate guanidinato(1–) ligands and thus expanded the scope of this ligand platform in transition-metal chemistry. Spectroscopic evidence suggests that the N,N-dialkyl-N',N''-diarylguanidinates employed here function as stronger donors than other monoanionic, nitrogen-based scaffolds, such as tris(pyrazolyl)borates-κ2 and β-diketiminates, when bound to the IrI(cod) unit. The trends observed in the reactions of these complexes with O2 illustrate the potential that the guanidinato(1–) platform has to offer for facile modulation of the reactivity of transition metal complexes.