

44099-GB6
Measuring Uptake Coefficients for Polycyclic Aromatic Hydrocarbons onto Aerosol Particles Using Photoionization Detection
Our research group has been active in characterizing the surface polarity and morphology of model tropospheric aerosol particles using probe molecule spectroscopy. A recent result of this work that was supported by the Petroleum Research Fund involves the surface morphology and phase behavior of model tropospheric particles. We study internally mixed aerosols made from salts (NaCl, KI) and the surfactant, sodium dodecyl sulfate (SDS), as models for particles of marine origin. At high values of relative humidity (RH), these particles exists as reverse micelles (aqueous salt core surrounded by a coating of the surfactant molecules), while at low RH, they comprise a solid core of salt coated with SDS and water. By measuring the photoelectric charging efficiency and the electronic spectroscopy of our probe molecule, coumarin 314 (C314), we detect two separate phase transitions in going from high to low RH. The first transition is the efflorescence of the aqueous salt core (45% RH for NaCl/SDS and 38% RH for KI/SDS), and this behavior is very similar to that for salt particles without a surfactant coating. Following this phase transition, a thin film of water and SDS remains on the surface. This thin film undergoes efflorescence at 5% RH. We also observe a hysteresis effect associated with this low RH phase transition such that the surface morphology of these particles depends on it history over a wide range of RH. Lastly, we find that this low RH phase transition is absent when the SDS mass percent (with respect to the dry particle) is below 3%. Using this threshold, we calculate the surface coverage of SDS must approach the saturated monolayer (~ 40 Å2 head group area) for the thin film to form.
 In
more recent work, we use this characterization of surfactant coated particles
to understand trends in the rate of uptake of polycyclic aromatic hydrocarbons
(PAHs) onto the particles' surface. In this experiment, the aerosol particle
stream interacts with a known pressure of PAH in a sliding injector flow tube. We monitor the surface concentration of PAH
as a function of particle-PAH interaction time using laser photoionization of
the surface-bound PAH. From the
resulting surface concentration vs. exposure time plot, we can determine the
initial uptake coefficient, g,
which is the initial uptake rate normalized to the gas-particle collision
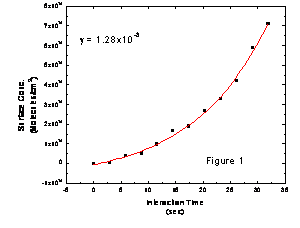
rate. Figure 1 shows some representative
data from the SDS-coated NaCl system.
(The curvature in the data arises from the loss of pyrene to the
flowtube wall, and we account for this loss in the data fitting
procedure.) The uptake coefficient for
SDS-coated NaCl is roughly an order of magnitude larger than for pure NaCl
particles.
In
more recent work, we use this characterization of surfactant coated particles
to understand trends in the rate of uptake of polycyclic aromatic hydrocarbons
(PAHs) onto the particles' surface. In this experiment, the aerosol particle
stream interacts with a known pressure of PAH in a sliding injector flow tube. We monitor the surface concentration of PAH
as a function of particle-PAH interaction time using laser photoionization of
the surface-bound PAH. From the
resulting surface concentration vs. exposure time plot, we can determine the
initial uptake coefficient, g,
which is the initial uptake rate normalized to the gas-particle collision
rate. Figure 1 shows some representative
data from the SDS-coated NaCl system.
(The curvature in the data arises from the loss of pyrene to the
flowtube wall, and we account for this loss in the data fitting
procedure.) The uptake coefficient for
SDS-coated NaCl is roughly an order of magnitude larger than for pure NaCl
particles.
 Figure 2 shows the dependence of the uptake
coefficient on the surface coverage of SDS for particles under dry conditions. For both 144 and 200 nm particles, g is near 1.5x10-4 for pure NaCl
particles. The uptake coefficient jumps
dramatically with nominally only 1 monolayer of SDS absorbed to the surface and
remains relatively constant at higher coverage.
Under dry conditions, our experiments suggest that SDS is not
necessarily evenly distributed on the surface; however the effect of the
surfactant is insensitive to the amount of SDS.
It is likely that the SDS domains increase in thickness as the nominal coverages increase rather than spreading out to cover more
of the surface.
Figure 2 shows the dependence of the uptake
coefficient on the surface coverage of SDS for particles under dry conditions. For both 144 and 200 nm particles, g is near 1.5x10-4 for pure NaCl
particles. The uptake coefficient jumps
dramatically with nominally only 1 monolayer of SDS absorbed to the surface and
remains relatively constant at higher coverage.
Under dry conditions, our experiments suggest that SDS is not
necessarily evenly distributed on the surface; however the effect of the
surfactant is insensitive to the amount of SDS.
It is likely that the SDS domains increase in thickness as the nominal coverages increase rather than spreading out to cover more
of the surface.
 One
of the central goes of our research is to correlate structural and
morphological data with dynamical properties.
As discussed above, the surfactant covered aerosol particles we have
studied retain a thin soapy film on the surface at low RH. To investigate the importance of this
morphological characteristic, we measured relative uptake coefficients for the
NaCl/SDS system over a wide range of RH.
We accomplish this by monitoring the photoelectric charging efficiency
(and, thus, the pyrene concentration) as we adjust the RH in the flow
tube. We carry out this experiment in
both a hydration and dehydration mode to highlight the important of morphology
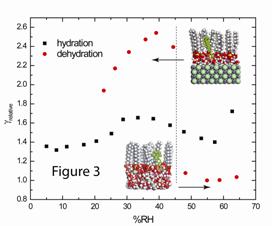
and hysteresis effects. Figure 3 shows
some of the data for the NaCl/SDS system.
The y-axis is the relative uptake coefficient normalized to the lowest
value (at 55% RH in the dehydration experiment). In the dehydration experiment, we find that
the lowest value of g corresponds the
aqueous morphology (>45% RH), and that g
increases by a factor of ~2.5 upon efflorescence. This feature changes with SDS coverage and is
most pronounced when the shrinking that accompanies the efflorescence of the
particle concentrates the SDS from sub-monolayer to super-monolayer coverages.
Interestingly, this thin film morphology is the most efficient in taking
up pyrene. In the hydration experiment, the uptake coefficient changes only
slightly with increasing RH as expected.
There is no phase change in this experiment and the particle morphology
is similar throughout. These
experiments highlight the important of particle morphology in working with
models of atmospheric particles.
One
of the central goes of our research is to correlate structural and
morphological data with dynamical properties.
As discussed above, the surfactant covered aerosol particles we have
studied retain a thin soapy film on the surface at low RH. To investigate the importance of this
morphological characteristic, we measured relative uptake coefficients for the
NaCl/SDS system over a wide range of RH.
We accomplish this by monitoring the photoelectric charging efficiency
(and, thus, the pyrene concentration) as we adjust the RH in the flow
tube. We carry out this experiment in
both a hydration and dehydration mode to highlight the important of morphology
and hysteresis effects. Figure 3 shows
some of the data for the NaCl/SDS system.
The y-axis is the relative uptake coefficient normalized to the lowest
value (at 55% RH in the dehydration experiment). In the dehydration experiment, we find that
the lowest value of g corresponds the
aqueous morphology (>45% RH), and that g
increases by a factor of ~2.5 upon efflorescence. This feature changes with SDS coverage and is
most pronounced when the shrinking that accompanies the efflorescence of the
particle concentrates the SDS from sub-monolayer to super-monolayer coverages.
Interestingly, this thin film morphology is the most efficient in taking
up pyrene. In the hydration experiment, the uptake coefficient changes only
slightly with increasing RH as expected.
There is no phase change in this experiment and the particle morphology
is similar throughout. These
experiments highlight the important of particle morphology in working with
models of atmospheric particles.