

47262-B6
Examining Hydrodynamic and Solubilization Properties of Micelles Formed by Chiral Amphiphiles with NMR
We have characterized the association of an atropisomeric binaphthyl compound (R,S-BNDHP) with aggregates of sodium cholate.
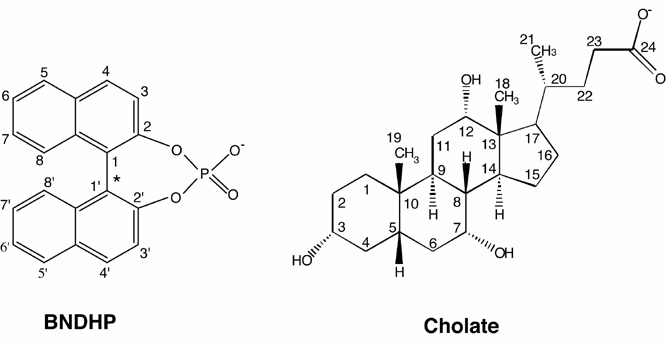
The relative orientation of the naphthyl planes about the ring bridging bond (indicated by the asterisk) in BNDHP prescribes non-superimposable structures corresponding to R,S-BNDHP. We have previously found through micellar electrokinetic capillary electrophoresis (MEKC) that R,S-BNDHP interact differentially with micelles formed from sodium cholate.
In our work to date, we have utilized NMR spectroscopy of R,S-BNDHP to (i) improve our understanding of the aggregation behavior of sodium cholate and (ii) identify regions of R,S-BNDHP that experience chirally selective interactions with the micelles.
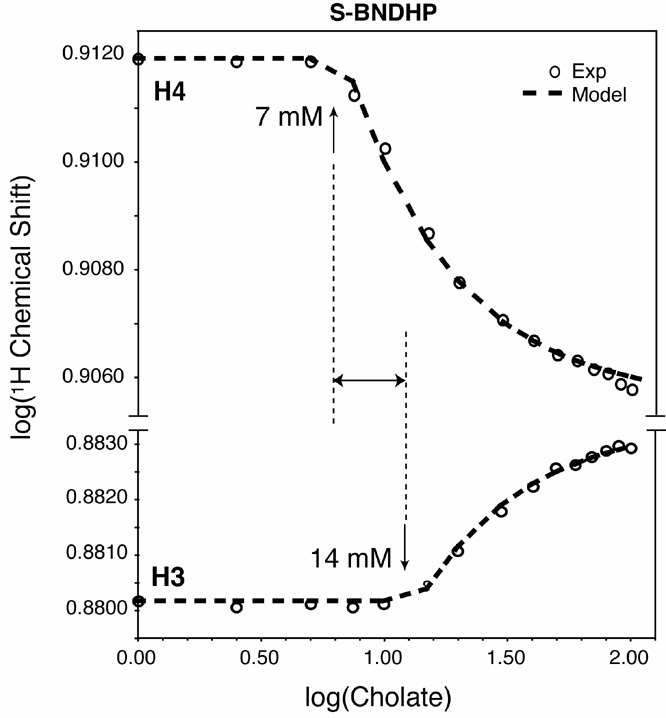
(i) Proton (1H) NMR of R,S-BNDHP unambiguously demonstrates the formation of a primary micelle at 15 mM cholate (pH 12), and a preliminary aggregate which forms at 7 mM cholate (figure below). The preliminary aggregate, which may be a dimer, contains a hydrophobic pocket that is sampled by BNDHP. Additional 1H and 31P chemical shift data (not shown) indicate the onset of progressive aggregation ca. 40-50 mM cholate (a.k.a. secondary aggregate). The chemical shifts of H3 of BNDHP are perturbed towards high frequency by association with cholate aggregates and support a widely suspected theory that secondary aggregates contain solvent-accessible hydrophilic surfaces.
(ii) Proton (1H) NMR has revealed that H4-H8 interact with a hydrophobic binding pocket in primary micelles of sodium cholate. H5,H6 and H7 experience differential interactions with the cholate micelles for the R and S-BNDHP forms and are therefore reporting on slightly different local environments within the cholate micelles.
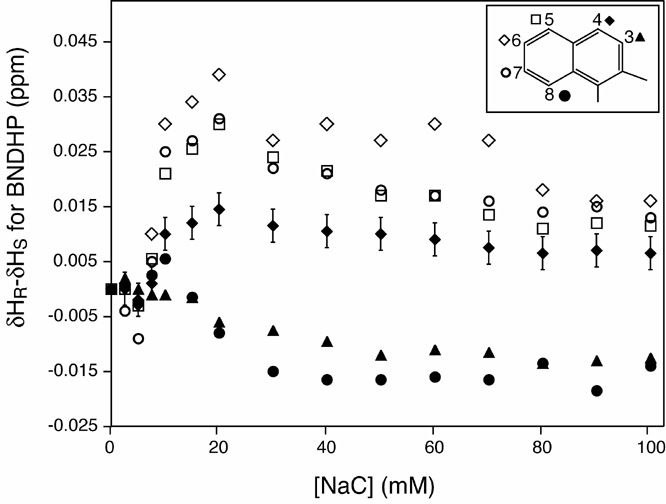
In other words, H5-H7 play a role in the chirally selective solvation of R,S-BNDHP by sodium cholate micelles. Further work is needed and underway to localize regions on cholate that also exhibit chirally selective interactions with R,S-BNDHP. The results to date are very encouraging progress towards better explaining the structural basis for bile salt mediated chiral solvation of hydrophobic analytes.